Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity
- PMID: 24781408
- PMCID: PMC4044307
- DOI: 10.14348/molcells.2014.0074
Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity
Abstract
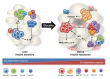
Recent findings, notably on adipokines and adipose tissue inflammation, have revised the concept of adipose tissues being a mere storage depot for body energy. Instead, adipose tissues are emerging as endocrine and immunologically active organs with multiple effects on the regulation of systemic energy homeostasis. Notably, compared with other metabolic organs such as liver and muscle, various inflammatory responses are dynamically regulated in adipose tissues and most of the immune cells in adipose tissues are involved in obesity-mediated metabolic complications, including insulin resistance. Here, we summarize recent findings on the key roles of innate (neutrophils, macrophages, mast cells, eosinophils) and adaptive (regulatory T cells, type 1 helper T cells, CD8 T cells, B cells) immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. In particular, the roles of natural killer T cells, one type of innate lymphocyte, in adipose tissue inflammation will be discussed. Finally, a new role of adipocytes as antigen presenting cells to modulate T cell activity and subsequent adipose tissue inflammation will be proposed.
Figures

References
-
- Bertola A., Ciucci T., Rousseau D., Bourlier V., Duffaut C., Bonnafous S., Blin-Wakkach C., Anty R., Iannelli A., Gugenheim J., et al. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes. 2012;61:2238–2247. - PMC - PubMed
-
- Bischoff S.C. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat. Rev. Immunol. 2007;7:93–104. - PubMed
-
- Bornstein S.R., Abu-Asab M., Glasow A., Path G., Hauner H., Tsokos M., Chrousos G.P., Scherbaum W.A. Immunohistochemical and ultrastructural localization of leptin and leptin receptor in human white adipose tissue and differentiating human adipose cells in primary culture. Diabetes. 2000;49:532–538. - PubMed
-
- Brennan P.J., Brigl M., Brenner M.B. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 2013;13:101–117. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

