Digital droplet PCR (ddPCR) for the precise quantification of human T-lymphotropic virus 1 proviral loads in peripheral blood and cerebrospinal fluid of HAM/TSP patients and identification of viral mutations
- PMID: 24781526
- PMCID: PMC4085507
- DOI: 10.1007/s13365-014-0249-3
Digital droplet PCR (ddPCR) for the precise quantification of human T-lymphotropic virus 1 proviral loads in peripheral blood and cerebrospinal fluid of HAM/TSP patients and identification of viral mutations
Abstract
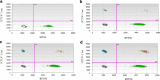
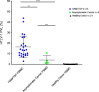
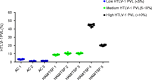
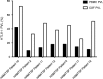
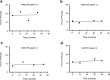
An elevated human T cell lymphotropic virus 1 (HTLV)-1 proviral load (PVL) is the main risk factor for developing HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) in HTLV-1 infected subjects, and a high cerebrospinal fluid (CSF) to peripheral blood mononuclear cell (PBMC) PVL ratio may be diagnostic of the condition. However, the standard method for quantification of HTLV-1 PVL-real-time PCR-has multiple limitations, including increased inter-assay variability in compartments with low cell numbers, such as CSF. Therefore, in this study, we evaluated a novel technique for HTVL-1 PVL quantification, digital droplet PCR (ddPCR). In ddPCR, PCR samples are partitioned into thousands of nanoliter-sized droplets, amplified on a thermocycler, and queried for fluorescent signal. Due to the high number of independent events (droplets), Poisson algorithms are used to determine absolute copy numbers independently of a standard curve, which enables highly precise quantitation. This assay has low intra-assay variability allowing for reliable PVL measurement in PBMC and CSF compartments of both asymptomatic carriers (AC) and HAM/TSP patients. It is also useful for HTLV-1-related clinical applications, such as longitudinal monitoring of PVL and identification of viral mutations within the region targeted by the primers and probe.
Figures


References
-
- Archanjo M, Batista, Fukutani, Bittencourt, Farre HTLV-1 proviral load levels in adult T-cell leukemia/lymphoma (ATL) in Bahia, Brazil. Retrovirology. 2011;8:A91. doi: 10.1186/1742-4690-8-S1-A91. - DOI
-
- Demontis MA, Hilburn S, Taylor GP. Human T cell lymphotropic virus type 1 viral load variability and long-term trends in asymptomatic carriers and in patients with human T cell lymphotropic virus type 1-related diseases. AIDS Res Hum Retrovir. 2013;29(2):359–364. - PubMed
-
- Furtado Mdos S, Andrade RG, Romanelli LC, Ribeiro MA, Ribas JG, Torres EB, Barbosa-Stancioli EF, Proietti AB, Martins ML. Monitoring the HTLV-1 proviral load in the peripheral blood of asymptomatic carriers and patients with HTLV-associated myelopathy/tropical spastic paraparesis from a Brazilian cohort: ROC curve analysis to establish the threshold for risk disease. J Med Virol. 2012;84(4):664–671. doi: 10.1002/jmv.23227. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

