In vitro resistance selections for Plasmodium falciparum dihydroorotate dehydrogenase inhibitors give mutants with multiple point mutations in the drug-binding site and altered growth
- PMID: 24782313
- PMCID: PMC4140291
- DOI: 10.1074/jbc.M114.558353
In vitro resistance selections for Plasmodium falciparum dihydroorotate dehydrogenase inhibitors give mutants with multiple point mutations in the drug-binding site and altered growth
Abstract
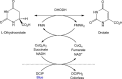
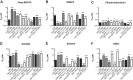
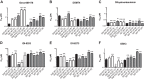
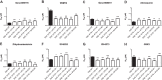
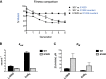
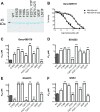
Malaria is a preventable and treatable disease; yet half of the world's population lives at risk of infection, and an estimated 660,000 people die of malaria-related causes every year. Rising drug resistance threatens to make malaria untreatable, necessitating both the discovery of new antimalarial agents and the development of strategies to identify and suppress the emergence and spread of drug resistance. We focused on in-development dihydroorotate dehydrogenase (DHODH) inhibitors. Characterizing resistance pathways for antimalarial agents not yet in clinical use will increase our understanding of the potential for resistance. We identified resistance mechanisms of Plasmodium falciparum (Pf) DHODH inhibitors via in vitro resistance selections. We found 11 point mutations in the PfDHODH target. Target gene amplification and unknown mechanisms also contributed to resistance, albeit to a lesser extent. These mutant parasites were often hypersensitive to other PfDHODH inhibitors, which immediately suggested a novel combination therapy approach to preventing resistance. Indeed, a combination of wild-type and mutant-type selective inhibitors led to resistance far less often than either drug alone. The effects of point mutations in PfDHODH were corroborated with purified recombinant wild-type and mutant-type PfDHODH proteins, which showed the same trends in drug response as the cognate cell lines. Comparative growth assays demonstrated that two mutant parasites grew less robustly than their wild-type parent, and the purified protein of those mutants showed a decrease in catalytic efficiency, thereby suggesting a reason for the diminished growth rate. Co-crystallography of PfDHODH with three inhibitors suggested that hydrophobic interactions are important for drug binding and selectivity.
Keywords: Drug Resistance; Evolution; Infectious Disease; Malaria; Nucleotide; Pyrimidine.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- World Health Organization (2012) World Malaria Report 2012 ( www.who.int/malaria/publications/world_malaria_report_2012/report/en/)
-
- Dondorp A. M., Nosten F., Yi P., Das D., Phyo A. P., Tarning J., Lwin K. M., Ariey F., Hanpithakpong W., Lee S. J., Ringwald P., Silamut K., Imwong M., Chotivanich K., Lim P., Herdman T., An S. S., Yeung S., Singhasivanon P., Day N. P., Lindegardh N., Socheat D., White N. J. (2009) Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361, 455–467 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases

