B cells promote obesity-associated periodontitis and oral pathogen-associated inflammation
- PMID: 24782490
- PMCID: PMC4101090
- DOI: 10.1189/jlb.4A0214-095R
B cells promote obesity-associated periodontitis and oral pathogen-associated inflammation
Abstract
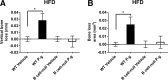
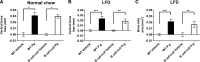
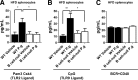
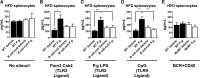
Individuals with T2D and PD suffer significantly from the ability of one disease to intensify the other. Disease-associated inflammation is one mechanism thought to fuel this pathogenic feed-forward loop. Several lines of evidence indicate that proinflammatory B cells promote T2D and PD; thus, B cells are top candidates for a cell type that predisposes PD in T2D. To test directly the role of B cells in T2D-associated PD, we compared outcomes from oral Porphyromonas gingivalis challenge of lean WT or B cell-null mice with outcomes from mice that were obese and insulin-resistant before challenge. Obese WT mice responded to oral P. gingivalis challenge with significant periodontal bone loss, whereas obese B cell-null mice were protected completely from PD. By contrast, lean WT and B cell-null mice suffer similar periodontal bone loss in response to oral pathogen. B cells from obese/insulin-resistant hosts also support oral osteoclastogenesis and both oral and systemic production of inflammatory cytokines, including pro-osteoclastogenic TNF-α and MIP-2, an ortholog of human IL-8. B cells furthermore impact AT inflammation in obese, P. gingivalis-infected hosts. Taken together, these data show that fundamentally different mechanisms regulate PD in lean and obese hosts, with B cells able to promote PD only if the hosts are "primed" by obesity. These results justify more intense analysis of obesity-associated changes in B cells that predispose PD in human T2D.
Keywords: B lymphocyte; Porphyromonas gingivalis; cytokine; mouse model; type 2 diabetes.
© 2014 Society for Leukocyte Biology.
Figures


References
-
- Vos T., Flaxman A. D., Naghavi M., Lozano R., Michaud C., Ezzati M., Shibuya K., Salomon J. A., Abdalla S., Aboyans V., et al. (2012) Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2163–2196 - PMC - PubMed
-
- Lockhart P. B., Bolger A. F., Papapanou P. N., Osinbowale O., Trevisan M., Levison M. E., Taubert K. A., Newburger J. W., Gornik H. L., Gewitz M. H., et al. (2012) Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association? A scientific statement from the American Heart Association. Circulation 125, 2520–2544 - PubMed
-
- Seymour G. J., Powell R. N., Davies W. I. (1979) The immunopathogenesis of progressive chronic inflammatory periodontal disease. J. Oral Pathol. 8, 249–265 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

