CD28-mediated pro-survival signaling induces chemotherapeutic resistance in multiple myeloma
- PMID: 24782505
- PMCID: PMC4055924
- DOI: 10.1182/blood-2013-10-530964
CD28-mediated pro-survival signaling induces chemotherapeutic resistance in multiple myeloma
Abstract
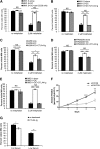
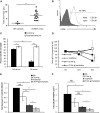
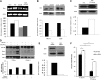
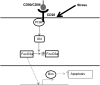
Chemotherapeutic resistance remains a significant hurdle in the treatment of multiple myeloma (MM) and is significantly mediated by interactions between MM cells and stromal cells of the bone marrow microenvironment. Despite the importance of these interactions, the specific molecules and downstream signaling components involved remain incompletely understood. We have previously shown that the prototypic T-cell costimulatory receptor CD28, which is also expressed on MM cells, is a key mediator of MM survival and apoptotic resistance. Crosslinking CD28 by agonistic antibodies or myeloid dendritic cells (DC; these express the CD28 ligands CD80/CD86) prevents apoptosis caused by chemotherapy or serum withdrawal. We now report that CD28 pro-survival signaling is dependent upon downstream activation of phosphatidyl-inositol 3-kinase/Akt, inactivation of the transcription factor FoxO3a, and decreased expression of the pro-apoptotic molecule Bim. Conversely, blocking the CD28-CD80/CD86 interaction between MM cells and DC in vitro abrogates the DC's ability to protect MM cells against chemotherapy-induced death. Consistent with these observations, in vivo blockade of CD28-CD80/CD86 in the Vk*MYC murine myeloma model sensitizes MM cells to chemotherapy and significantly reduces tumor burden. Taken together, our findings suggest that CD28 is an important mediator of MM survival during stress and can be targeted to overcome chemotherapy resistance.
© 2014 by The American Society of Hematology.
Figures


References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. - PubMed
-
- Attal M, Harousseau JL, Leyvraz S, et al. Inter-Groupe Francophone du Myélome (IFM) Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108(10):3289–3294. - PubMed
-
- Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609–2617. - PubMed
-
- Spanswick VJ, Craddock C, Sekhar M, et al. Repair of DNA interstrand crosslinks as a mechanism of clinical resistance to melphalan in multiple myeloma. Blood. 2002;100(1):224–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

