Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15
- PMID: 24782509
- PMCID: PMC4055922
- DOI: 10.1182/blood-2014-01-552174
Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15
Abstract
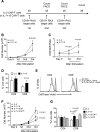
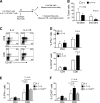
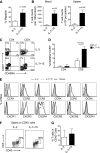
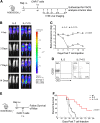
Adoptive transfer of T lymphocytes expressing a CD19-specific chimeric antigen receptor (CAR.CD19) induces complete tumor regression in patients with lymphoid malignancies. Although in vivo persistence of CAR-T cells correlates with clinical responses, it remains unknown whether specific cell subsets within the CAR-T-cell product correlate with their subsequent in vivo expansion and persistence. We analyzed 14 patients with B-cell malignancies infused with autologous CAR.CD19-redirected T cells expanded ex vivo using IL-2, and found that their in vivo expansion only correlated with the frequency within the infused product of a CD8(+)CD45RA(+)CCR7(+) subset, whose phenotype is closest to "T-memory stem cells." Preclinical models showed that increasing the frequency of CD8(+)CD45RA(+)CCR7(+) CAR-T cells in the infused line by culturing the cells with IL-7 and IL-15 produced greater antitumor activity of CAR-T cells mediated by increased resistance to cell death, following repetitive encounters with the antigen, while preserving their migration to secondary lymphoid organs. This trial was registered at www.clinicaltrials.gov as #NCT00586391 and #NCT00709033.
© 2014 by The American Society of Hematology.
Figures



References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

