Role of pancreatic stellate cells in chemoresistance in pancreatic cancer
- PMID: 24782785
- PMCID: PMC3988387
- DOI: 10.3389/fphys.2014.00141
Role of pancreatic stellate cells in chemoresistance in pancreatic cancer
Abstract
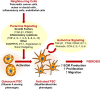
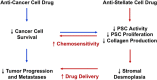
Pancreatic cancer is highly chemoresistant. A major contributing factor is the characteristic extensive stromal or fibrotic reaction, which comprises up to 90% of the tumor volume. Over the last decade there has been intensive research into the role of the pro-fibrogenic pancreatic stellate cells (PSCs) and their interaction with pancreatic cancer cells. As a result of the significant alterations in the tumor microenvironment following activation of PSCs, tumor progression, and chemoresistance is enhanced. This review will discuss how PSCs contribute to chemoresistance in pancreatic cancer.
Keywords: chemoresistance; fibrosis; hypoxia; pancreatic cancer; pancreatic stellate cells; stroma.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

