Effect of the soil type on the microbiome in the rhizosphere of field-grown lettuce
- PMID: 24782839
- PMCID: PMC3986527
- DOI: 10.3389/fmicb.2014.00144
Effect of the soil type on the microbiome in the rhizosphere of field-grown lettuce
Abstract
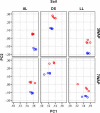
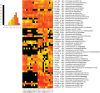
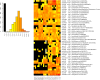
The complex and enormous diversity of microorganisms associated with plant roots is important for plant health and growth and is shaped by numerous factors. This study aimed to unravel the effects of the soil type on bacterial communities in the rhizosphere of field-grown lettuce. We used an experimental plot system with three different soil types that were stored at the same site for 10 years under the same agricultural management to reveal differences directly linked to the soil type and not influenced by other factors such as climate or cropping history. Bulk soil and rhizosphere samples were collected 3 and 7 weeks after planting. The analysis of 16S rRNA gene fragments amplified from total community DNA by denaturing gradient gel electrophoresis and pyrosequencing revealed soil type dependent differences in the bacterial community structure of the bulk soils and the corresponding rhizospheres. The rhizosphere effect differed depending on the soil type and the plant growth developmental stage. Despite the soil type dependent differences in the bacterial community composition several genera such as Sphingomonas, Rhizobium, Pseudomonas, and Variovorax were significantly increased in the rhizosphere of lettuce grown in all three soils. The number of rhizosphere responders was highest 3 weeks after planting. Interestingly, in the soil with the highest numbers of responders the highest shoot dry weights were observed. Heatmap analysis revealed that many dominant operational taxonomic units were shared among rhizosphere samples of lettuce grown in diluvial sand, alluvial loam, and loess loam and that only a subset was increased in relative abundance in the rhizosphere compared to the corresponding bulk soil. The findings of the study provide insights into the effect of soil types on the rhizosphere microbiome of lettuce.
Keywords: 16S rRNA gene analysis; DGGE; Lactuca sativa; bacterial communities; pyrosequencing; rhizosphere responders.
Figures

References
-
- Adesina M. F., Grosch R., Lembke A., Vatchev T. D., Smalla K. (2009). In vitro antagonists of Rhizoctonia solani tested on lettuce: rhizosphere competence, biocontrol efficiency and rhizosphere microbial community response. FEMS Microbiol. Ecol. 69, 62–74 10.1111/j.1574-6941.2009.00685.x - DOI - PubMed
-
- Baudoin E., Benizri E., Guckert A. (2002). Impact of growth stage on the bacterial community structure along maize roots, as determined by metabolic and genetic fingerprinting. Appl. Soil Ecol. 19, 135–145 10.1016/S0929-1393(01)00185-8 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

