Paradigm Shift in Dendritic Cell-Based Immunotherapy: From in vitro Generated Monocyte-Derived DCs to Naturally Circulating DC Subsets
- PMID: 24782868
- PMCID: PMC3990057
- DOI: 10.3389/fimmu.2014.00165
Paradigm Shift in Dendritic Cell-Based Immunotherapy: From in vitro Generated Monocyte-Derived DCs to Naturally Circulating DC Subsets
Abstract
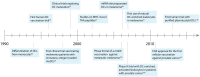
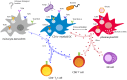
Dendritic cell (DC)-based immunotherapy employs the patients' immune system to fight neoplastic lesions spread over the entire body. This makes it an important therapy option for patients suffering from metastatic melanoma, which is often resistant to chemotherapy. However, conventional cellular vaccination approaches, based on monocyte-derived DCs (moDCs), only achieved modest response rates despite continued optimization of various vaccination parameters. In addition, the generation of moDCs requires extensive ex vivo culturing conceivably hampering the immunogenicity of the vaccine. Recent studies, thus, focused on vaccines that make use of primary DCs. Though rare in the blood, these naturally circulating DCs can be readily isolated and activated thereby circumventing lengthy ex vivo culture periods. The first clinical trials not only showed increased survival rates but also the induction of diversified anti-cancer immune responses. Upcoming treatment paradigms aim to include several primary DC subsets in a single vaccine as pre-clinical studies identified synergistic effects between various antigen-presenting cells.
Keywords: dendritic cell vaccination; immunotherapy; melanoma; monocyte-derived dendritic cells; myeloid dendritic cells; naturally circulating dendritic cells; plasmacytoid dendritic cells.
Figures
References
-
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Web Page]. Lyon: International Agency for Research on Cancer; (2012).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources