How Do CD4(+) T Cells Detect and Eliminate Tumor Cells That Either Lack or Express MHC Class II Molecules?
- PMID: 24782871
- PMCID: PMC3995058
- DOI: 10.3389/fimmu.2014.00174
How Do CD4(+) T Cells Detect and Eliminate Tumor Cells That Either Lack or Express MHC Class II Molecules?
Abstract
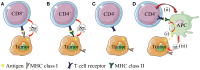
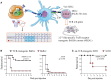
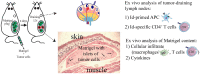
CD4(+) T cells contribute to tumor eradication, even in the absence of CD8(+) T cells. Cytotoxic CD4(+) T cells can directly kill MHC class II positive tumor cells. More surprisingly, CD4(+) T cells can indirectly eliminate tumor cells that lack MHC class II expression. Here, we review the mechanisms of direct and indirect CD4(+) T cell-mediated elimination of tumor cells. An emphasis is put on T cell receptor (TCR) transgenic models, where anti-tumor responses of naïve CD4(+) T cells of defined specificity can be tracked. Some generalizations can tentatively be made. For both MHCII(POS) and MHCII(NEG) tumors, presentation of tumor-specific antigen by host antigen-presenting cells (APCs) appears to be required for CD4(+) T cell priming. This has been extensively studied in a myeloma model (MOPC315), where host APCs in tumor-draining lymph nodes are primed with secreted tumor antigen. Upon antigen recognition, naïve CD4(+) T cells differentiate into Th1 cells and migrate to the tumor. At the tumor site, the mechanisms for elimination of MHCII(POS) and MHCII(NEG) tumor cells differ. In a TCR-transgenic B16 melanoma model, MHCII(POS) melanoma cells are directly killed by cytotoxic CD4(+) T cells in a perforin/granzyme B-dependent manner. By contrast, MHCII(NEG) myeloma cells are killed by IFN-γ stimulated M1-like macrophages. In summary, while the priming phase of CD4(+) T cells appears similar for MHCII(POS) and MHCII(NEG) tumors, the killing mechanisms are different. Unresolved issues and directions for future research are addressed.
Keywords: CD4+ T cells; MHC class II; T cell receptor transgenic; T helper 1; multiple myeloma; transgenic mouse models; tumor antigen; tumor immunology.
Figures

References
-
- Thomas L. Discussion in Cellular and Humoral Aspects of the Hypersensitive State. New York: Hoebner-Harper; (1959). p. 529–32
-
- Burnet FM. The evolution of bodily defence. Med J Aust (1963) 2:817–21 - PubMed
-
- Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res (1970) 13:1–27 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

