Targeted Disruption of the JAK2/STAT3 Pathway in Combination with Systemic Administration of Paclitaxel Inhibits the Priming of Ovarian Cancer Stem Cells Leading to a Reduced Tumor Burden
- PMID: 24782986
- PMCID: PMC3988380
- DOI: 10.3389/fonc.2014.00075
Targeted Disruption of the JAK2/STAT3 Pathway in Combination with Systemic Administration of Paclitaxel Inhibits the Priming of Ovarian Cancer Stem Cells Leading to a Reduced Tumor Burden
Abstract
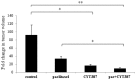
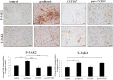
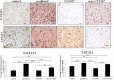
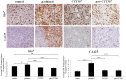
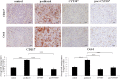
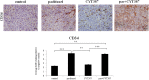
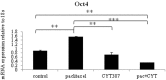
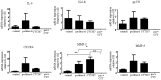
Chemotherapy resistance associated with recurrent disease is the major cause of poor survival of ovarian cancer patients. We have recently demonstrated activation of the JAK2/STAT3 pathway and the enhancement of a cancer stem cell (CSC)-like phenotype in ovarian cancer cells treated in vitro with chemotherapeutic agents. To elucidate further these mechanisms in vivo, we used a two-tiered paclitaxel treatment approach in nude mice inoculated with ovarian cancer cells. In the first approach, we demonstrate that a single intraperitoneal administration of paclitaxel in mice 7 days after subcutaneous transplantation of the HEY ovarian cancer cell line resulted in a significant increase in the expression of CA125, Oct4, and CD117 in mice xenografts compared to control mice xenografts which did not receive paclitaxel. In the second approach, mice were administered once weekly with paclitaxel and/or a daily dose of the JAK2-specific inhibitor, CYT387, over 4 weeks. Mice receiving paclitaxel only demonstrated a significant decrease in tumor volume compared to control mice. At the molecular level, mouse tumors remaining after paclitaxel administration showed a significant increase in the expression of Oct4 and CD117 coinciding with a significant activation of the JAK2/STAT3 pathway compared to control tumors. The addition of CYT387 with paclitaxel resulted in the suppression of JAK2/STAT3 activation and abrogation of Oct4 and CD117 expression in mouse xenografts. This coincided with significantly smaller tumors in mice administered CYT387 in addition to paclitaxel, compared to the control group and the group of mice receiving paclitaxel only. These data suggest that the systemic administration of paclitaxel enhances Oct4- and CD117-associated CSC-like marker expression in surviving cancer cells in vivo, which can be suppressed by the addition of the JAK2-specific inhibitor CYT387, leading to a significantly smaller tumor burden. These novel findings have the potential for the development of CSC-targeted therapy to improve the treatment outcomes of ovarian cancer patients.
Keywords: JAK2/STAT3 pathway; cancer stem cells; chemoresistance; metastasis; ovarian carcinoma; recurrence.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

