Treatment of rheumatoid arthritis with biologic DMARDS (Rituximab and Etanercept)
- PMID: 24783914
- PMCID: PMC4272488
- DOI: 10.5455/medarh.2014.68.51-53
Treatment of rheumatoid arthritis with biologic DMARDS (Rituximab and Etanercept)
Abstract
Goal: To determine efficacy and safety of treatment with Rituximab and Etanercept plus Methotrexate in patients with active Rheumatoid Arthritis (RA), who had an inadequate response to nonbiologic DMARDS therapies and to explore the pharmacogenetics and pharmacodynamics of Rituximab and Etanercept in our populations. Study was done at Rheumatology Clinic of University Clinical Centre in Prishtina during 2009-2011 years.
Methods: We evaluated primary efficacy and safety at 24 weeks in patients enrolled in the study of long-term efficacy of Rituximab and Etanercept. Patients with active Rheumatoid Arthritis and an inadequate response to 1 or more non biologic DMARDS were randomized to receive intravenous Rituximab (1 course consisting of 2 infusions of 1.000 mg each -one group, and Etanercept 25 mg twice weekly -second group, but both groups with background MTX. The primary efficacy end point was a response on the ACR 20%, improvement criteria at 24 weeks, Secondary end points were responses on the ACR 50 and ACR 70, improvement criteria, the DAS 28, and EULAR response criteria at 24 weeks.
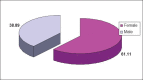
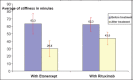
Results: During our investigations we treated 20 patients, 15 females and 5 males, in the treated group with RTX and 13 patients 8 females and 5 males in the treated group with ETN. Patients of group 1 and group 2 were of ages 37-69 years old and 19-69 years old (average 47-44) Most of the patients belong in 2nd and 3rd functional stage according to Steinbrocker. All ACR response parameters were significantly improved in RTX treated patients who also had clinically meaningful improvement in fatigue, disability and quality of life. Patients showed a trend less progression in radiographic end points. Most adverse events occurred with the first RTX infusion and were mild to moderate severity.
Conclusion: At 24 weeks, a single course of RTX and ETN provided significant and clinically meaningful improvements in disease activity in patients with active, longstanding RA who had an inadequate response to 1 or more nonbiologic DMARDS.
Conflict of interest statement
Figures
References
-
- Lange Medical Books. 2nd edition. Vol. 67. New York, Chicago, San Francisco: Mc Graw Hill, Medical Publishing Division; 2007. Lange. Current diagnosis and treatment on Rheumatology; pp. 522–532.
-
- Hochberg MC, Chane RW, Dwosh I, Lindsay S, Pincus T, Wolfe F. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum. 1992;25:498–502. - PubMed
-
- Firestein . 8th edition. Vol. 52. WB Saunders Company; 2008. Kelly textbook of Rheumatology; p. 330.
-
- Lange . 2nd edition. Vol. 3. New York, Chicago; San Francisco: Medical Graw Hill; 2011. Basic Radiology; pp. 155–163.
-
- Van Riel PLCM. Provisional guidelines for measuring disease activity in clinical trials on RA. Br. J Rheumatol. 1992;31:793–796. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical