Natural compounds as next-generation herbicides
- PMID: 24784133
- PMCID: PMC4226356
- DOI: 10.1104/pp.114.239061
Natural compounds as next-generation herbicides
Abstract
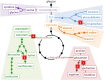
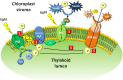
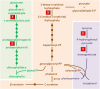
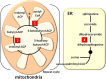
Herbicides with new modes of action (MOAs) are badly needed due to the rapidly evolving resistance to commercial herbicides, but a new MOA has not been introduced in over 20 years. The greatest pest management challenge for organic agriculture is the lack of effective natural product herbicides. The structural diversity and evolved biological activity of natural phytotoxins offer opportunities for the development of both directly used natural compounds and synthetic herbicides with new target sites based on the structures of natural phytotoxins. Natural phytotoxins are also a source for the discovery of new herbicide target sites that can serve as the focus of traditional herbicide discovery efforts. There are many examples of strong natural phytotoxins with MOAs other than those used by commercial herbicides, which indicates that there are molecular targets of herbicides that can be added to the current repertoire of commercial herbicide MOAs.
© 2014 American Society of Plant Biologists. All Rights Reserved.
Figures
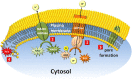
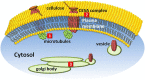
References
-
- Abbas HK, Duke SO, Shier WT, Duke MV (2002) Inhibition of ceramide synthesis in plants by phytotoxins. In RK Upadhyay, ed, Advances in Microbial Toxin Research and Its Biotechnological Exploitation. Kluwer Academic/Plenum, London, pp 211–229
-
- Abbas HK, Gronwald JW, Plaisance KL, Paul RN, Lee YW. (2001) Histone deacetylase activity and phytotoxic effects following exposure of Duckweed (Lemna pausicostata L.) to apicidin and HC-toxin. Phytopathology 91: 1141–1148 - PubMed
-
- Abbas HK, Paul RN, Riley RT, Tanaka T, Shier WT. (1998) Ultrastructural effects of AAL-toxin TA from the fungus Alternaria alternata on black nightshade (Solanum nigrum L.) leaf discs and correlation with biochemical measures of toxicity. Toxicon 36: 1821–1832 - PubMed
-
- Abbas HK, Tanaka T, Duke SO, Boyette CD. (1995) Susceptibility of various crop and weed species to AAL-toxin, a natural herbicide. Weed Technol 9: 125–130
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

