A UDP-Glucose:Monoterpenol Glucosyltransferase Adds to the Chemical Diversity of the Grapevine Metabolome
- PMID: 24784757
- PMCID: PMC4044836
- DOI: 10.1104/pp.113.232470
A UDP-Glucose:Monoterpenol Glucosyltransferase Adds to the Chemical Diversity of the Grapevine Metabolome
Abstract
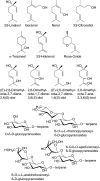
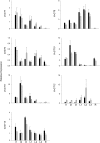
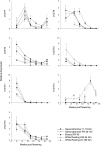
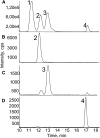
Terpenoids represent one of the major classes of natural products and serve different biological functions. In grape (Vitis vinifera), a large fraction of these compounds is present as nonvolatile terpene glycosides. We have extracted putative glycosyltransferase (GT) sequences from the grape genome database that show similarity to Arabidopsis (Arabidopsis thaliana) GTs whose encoded proteins glucosylate a diversity of terpenes. Spatial and temporal expression levels of the potential VvGT genes were determined in five different grapevine varieties. Heterologous expression and biochemical assays of candidate genes led to the identification of a UDP-glucose:monoterpenol β-d-glucosyltransferase (VvGT7). The VvGT7 gene was expressed in various tissues in accordance with monoterpenyl glucoside accumulation in grape cultivars. Twelve allelic VvGT7 genes were isolated from five cultivars, and their encoded proteins were biochemically analyzed. They varied in substrate preference and catalytic activity. Three amino acids, which corresponded to none of the determinants previously identified for other plant GTs, were found to be important for enzymatic catalysis. Site-specific mutagenesis along with the analysis of allelic proteins also revealed amino acids that impact catalytic activity and substrate tolerance. These results demonstrate that VvGT7 may contribute to the production of geranyl and neryl glucoside during grape ripening.
© 2014 American Society of Plant Biologists. All Rights Reserved.
Figures




References
-
- Baek HH, Cadwallader KR. (1999) Contribution of free and glycosidically bound volatile compounds to the aroma of Muscadine grape juice. J Food Sci 64: 441–444
-
- Bayrak A. (1994) Volatile oil composition of Turkish rose (Rosa damascena). J Agric Food Chem 64: 441–448
-
- Bowles D, Lim EK, Poppenberger B, Vaistij FE. (2006) Glycosyltransferases of lipophilic small molecules. Annu Rev Plant Biol 57: 567–597 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

