Heritable transmission of stress resistance by high dietary glucose in Caenorhabditis elegans
- PMID: 24785260
- PMCID: PMC4006733
- DOI: 10.1371/journal.pgen.1004346
Heritable transmission of stress resistance by high dietary glucose in Caenorhabditis elegans
Abstract
Glucose is a major energy source and is a key regulator of metabolism but excessive dietary glucose is linked to several disorders including type 2 diabetes, obesity and cardiac dysfunction. Dietary intake greatly influences organismal survival but whether the effects of nutritional status are transmitted to the offspring is an unresolved question. Here we show that exposing Caenorhabditis elegans to high glucose concentrations in the parental generation leads to opposing negative effects on fecundity, while having protective effects against cellular stress in the descendent progeny. The transgenerational inheritance of glucose-mediated phenotypes is dependent on the insulin/IGF-like signalling pathway and components of the histone H3 lysine 4 trimethylase complex are essential for transmission of inherited phenotypes. Thus dietary over-consumption phenotypes are heritable with profound effects on the health and survival of descendants.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
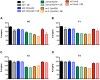

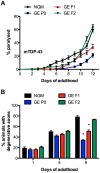


References
-
- Katz DJ, Edwards TM, Reinke V, Kelly WG (2009) A C. elegans LSD1 demethylase contributes to germline immortality by reprogramming epigenetic memory. Cell 137: 308–320 Available: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id.... - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

