Early-onset group B streptococcal disease in the United States: potential for further reduction
- PMID: 24785612
- PMCID: PMC11863723
- DOI: 10.1097/AOG.0000000000000163
Early-onset group B streptococcal disease in the United States: potential for further reduction
Abstract
Objective: To describe lapses in adherence to group B streptococcus (GBS) prevention guidelines among cases of early-onset GBS disease in term and preterm neonates and to estimate the potential for further reduction in disease burden under current prevention strategies.
Methods: We reviewed labor and delivery and prenatal records of mothers of neonates with early-onset GBS disease (aged younger than 7 days with GBS isolated from a normally sterile site) identified at population-based surveillance sites in 2008-2009. We interviewed prenatal care providers about GBS screening practices and obtained relevant laboratory records. We evaluated the data for errors in prenatal screening, laboratory methods, communication of results, and intrapartum antibiotic prophylaxis. Using published data on screening sensitivity and intrapartum prophylaxis effectiveness, we estimated the potential reduction in cases under optimal prevention implementation.
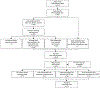
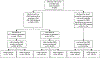
Results: Among 309 cases, 179 (57.9%) had one or more implementation errors. The most common error type in term and preterm case-patients was prenatal screening (80 of 222 [36.0%]) and intrapartum prophylaxis (46 of 85 [54.1%]), respectively. We estimated that under optimal implementation, cases of early-onset GBS disease could be reduced by 26-59% with the largest benefit from a single intervention coming from improved use of intrapartum prophylaxis (16% decrease).
Conclusion: Further reduction of early-onset GBS disease burden is possible under current prevention strategies, particularly with improved implementation of antibiotic prophylaxis. However, even with perfect adherence to recommended practices, the decline in cases may be modest. Therefore, novel prevention approaches such as improved intrapartum assays and vaccines are also needed.
Conflict of interest statement
Financial Disclosure
The authors did not report any potential conflicts of interest.
Figures
References
-
- Verani JR, McGee L, Schrag SJ; Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm Rep 2010;59:1–36. - PubMed
-
- Centers for Disease Control and Prevention. Active bacterial core surveillance report, emerging infections program network, group B streptococcus, 2008. 2009. Available at: http://www.cdc.gov/abcs/reports-findings/survreports/gbs08.html. Retrieved May 6, 2013.
-
- Centers for Disease Control and Prevention. Active bacterial core surveillance report, emerging infections program network, group B streptococcus, 2009. 2010. Available at: http://www.cdc.gov/abcs/reports-findings/survreports/gbs09.html. Retrieved May 6, 2013.
-
- Centers for Disease Control and Prevention. Active bacterial core surveillance report, emerging infections program network, group B streptococcus, 2010. 2011. Available at: http://www.cdc.gov/abcs/reports-findings/survreports/gbs10.html. Retrieved May 6, 2013.
-
- Centers for Disease Control and Prevention. Active bacterial core surveillance report, emerging infections program network, group B streptococcus, 2011. 2012. Available at: http://www.cdc.gov/abcs/reports-findings/survreports/gbs11.html. Retrieved May 6, 2013.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

