Chemical inhibition of NAT10 corrects defects of laminopathic cells
- PMID: 24786082
- PMCID: PMC4246063
- DOI: 10.1126/science.1252651
Chemical inhibition of NAT10 corrects defects of laminopathic cells
Abstract
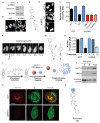
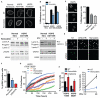
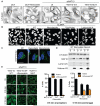
Down-regulation and mutations of the nuclear-architecture proteins lamin A and C cause misshapen nuclei and altered chromatin organization associated with cancer and laminopathies, including the premature-aging disease Hutchinson-Gilford progeria syndrome (HGPS). Here, we identified the small molecule "Remodelin" that improved nuclear architecture, chromatin organization, and fitness of both human lamin A/C-depleted cells and HGPS-derived patient cells and decreased markers of DNA damage in these cells. Using a combination of chemical, cellular, and genetic approaches, we identified the acetyl-transferase protein NAT10 as the target of Remodelin that mediated nuclear shape rescue in laminopathic cells via microtubule reorganization. These findings provide insights into how NAT10 affects nuclear architecture and suggest alternative strategies for treating laminopathies and aging.
Figures

References
-
- De Sandre-Giovannoli A, et al. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003 Jun 27;300:2055. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

