Molecular mechanisms and new treatment strategies for non-alcoholic steatohepatitis (NASH)
- PMID: 24786095
- PMCID: PMC4057677
- DOI: 10.3390/ijms15057352
Molecular mechanisms and new treatment strategies for non-alcoholic steatohepatitis (NASH)
Abstract
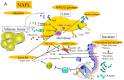
Non-alcoholic steatohepatitis (NASH) is a severe form of non-alcoholic fatty liver disease (NAFLD), in which most patients exhibit non-progressive, non-alcoholic fatty liver (NAFL) attributable to simple steatosis. Multiple hits, including genetic differences, fat accumulation, insulin resistance and intestinal microbiota changes, account for the progression of NASH. NAFLD is strongly associated with obesity, which induces adipokine secretion, endoplasmic reticulum (ER) and oxidative stress at the cellular level, which in turn induces hepatic steatosis, inflammation and fibrosis. Among these factors, gut microbiota are acknowledged as having an important role in initiating this multifactorial disease. Oxidative stress is considered to be a key contributor in the progression from NAFL to NASH. Macrophage infiltration is apparent in NAFL and NASH, while T-cell infiltration is apparent in NASH. Although several clinical trials have shown that antioxidative therapy with vitamin E can effectively control hepatitis pathology in the short term, the long-term effects remain obscure and have often proved to be ineffective in many other diseases. Several long-term antioxidant protocols have failed to reduce mortality. New treatment modalities that incorporate current understanding of NAFLD molecular pathogenesis must be considered.
Figures


Similar articles
-
Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH).Int J Mol Sci. 2013 Oct 15;14(10):20704-28. doi: 10.3390/ijms141020704. Int J Mol Sci. 2013. PMID: 24132155 Free PMC article. Review.
-
CD44 is a key player in non-alcoholic steatohepatitis.J Hepatol. 2017 Aug;67(2):328-338. doi: 10.1016/j.jhep.2017.03.003. Epub 2017 Mar 16. J Hepatol. 2017. PMID: 28323124
-
Vitamin E alleviates non-alcoholic fatty liver disease in phosphatidylethanolamine N-methyltransferase deficient mice.Biochim Biophys Acta Mol Basis Dis. 2019 Jan;1865(1):14-25. doi: 10.1016/j.bbadis.2018.10.010. Epub 2018 Oct 6. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 30300671
-
Progression of non-alcoholic steatosis to steatohepatitis and fibrosis parallels cumulative accumulation of danger signals that promote inflammation and liver tumors in a high fat-cholesterol-sugar diet model in mice.J Transl Med. 2015 Jun 16;13:193. doi: 10.1186/s12967-015-0552-7. J Transl Med. 2015. PMID: 26077675 Free PMC article.
-
Metabolic drivers of non-alcoholic fatty liver disease.Mol Metab. 2021 Aug;50:101143. doi: 10.1016/j.molmet.2020.101143. Epub 2020 Dec 17. Mol Metab. 2021. PMID: 33346069 Free PMC article. Review.
Cited by
-
Bitter melon extract ameliorates palmitate-induced apoptosis via inhibition of endoplasmic reticulum stress in HepG2 cells and high-fat/high-fructose-diet-induced fatty liver.Food Nutr Res. 2018 Mar 22;62. doi: 10.29219/fnr.v62.1319. eCollection 2018. Food Nutr Res. 2018. PMID: 30026676 Free PMC article.
-
Oxidative Stress Management in Chronic Liver Diseases and Hepatocellular Carcinoma.Nutrients. 2020 May 28;12(6):1576. doi: 10.3390/nu12061576. Nutrients. 2020. PMID: 32481552 Free PMC article. Review.
-
Berberine prevents progression from hepatic steatosis to steatohepatitis and fibrosis by reducing endoplasmic reticulum stress.Sci Rep. 2016 Feb 9;6:20848. doi: 10.1038/srep20848. Sci Rep. 2016. PMID: 26857750 Free PMC article.
-
Effects of 4-nonylphenol on oxidant/antioxidant balance system inducing hepatic steatosis in male rat.Toxicol Rep. 2015 Oct 19;2:1423-1433. doi: 10.1016/j.toxrep.2015.10.006. eCollection 2015. Toxicol Rep. 2015. PMID: 28962484 Free PMC article.
-
Combined Esculentin-2CHa Fusion Protein-Coated Au Nanoparticles for Effective Against Non-Alcoholic Fatty Liver Disease in Mice Model.Int J Nanomedicine. 2025 Mar 17;20:3407-3421. doi: 10.2147/IJN.S497645. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40125429 Free PMC article.
References
-
- Pacifico L., Anania C., Martino F., Poggiogalle E., Chiarelli F., Arca M., Chiesa C. Management of metabolic syndrome in children and adolescents. Nutr. Metab. Cardiovasc. Dis. 2011;21:455–466. - PubMed
-
- Matteoni C.A., Younossi Z.M., Gramlich T., Boparai N., Liu Y.C., McCullough A.J. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. - PubMed
-
- Brunt E.M., Kleiner D.E., Wilson L.A., Unalp A., Behling C.E., Lavine J.E., Neuschwander-Tetri B.A. Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): A histologic marker of advanced NAFLD-Clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49:809–820. - PMC - PubMed
-
- Yatsuji S., Hashimoto E., Tobari M., Taniai M., Tokushige K., Shiratori K. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J. Gastroenterol. Hepatol. 2009;24:248–254. - PubMed
-
- Hatanaka K., Kudo M., Fukunaga T., Ueshima K., Chung H., Minami Y., Sakaguchi Y., Hagiwara S., Orino A., Osaki Y. Clinical characteristics of NonBNonC-HCC: Comparison with HBV and HCV related HCC. Intervirology. 2007;50:24–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

