Genome-wide profiling of the cardiac transcriptome after myocardial infarction identifies novel heart-specific long non-coding RNAs
- PMID: 24786300
- PMCID: PMC4320320
- DOI: 10.1093/eurheartj/ehu180
Genome-wide profiling of the cardiac transcriptome after myocardial infarction identifies novel heart-specific long non-coding RNAs
Abstract
Aim: Heart disease is recognized as a consequence of dysregulation of cardiac gene regulatory networks. Previously, unappreciated components of such networks are the long non-coding RNAs (lncRNAs). Their roles in the heart remain to be elucidated. Thus, this study aimed to systematically characterize the cardiac long non-coding transcriptome post-myocardial infarction and to elucidate their potential roles in cardiac homoeostasis.
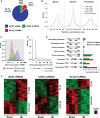
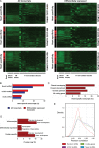
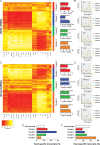
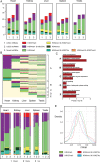
Methods and results: We annotated the mouse transcriptome after myocardial infarction via RNA sequencing and ab initio transcript reconstruction, and integrated genome-wide approaches to associate specific lncRNAs with developmental processes and physiological parameters. Expression of specific lncRNAs strongly correlated with defined parameters of cardiac dimensions and function. Using chromatin maps to infer lncRNA function, we identified many with potential roles in cardiogenesis and pathological remodelling. The vast majority was associated with active cardiac-specific enhancers. Importantly, oligonucleotide-mediated knockdown implicated novel lncRNAs in controlling expression of key regulatory proteins involved in cardiogenesis. Finally, we identified hundreds of human orthologues and demonstrate that particular candidates were differentially modulated in human heart disease.
Conclusion: These findings reveal hundreds of novel heart-specific lncRNAs with unique regulatory and functional characteristics relevant to maladaptive remodelling, cardiac function and possibly cardiac regeneration. This new class of molecules represents potential therapeutic targets for cardiac disease. Furthermore, their exquisite correlation with cardiac physiology renders them attractive candidate biomarkers to be used in the clinic.
Keywords: Heart failure; Long non-coding RNAs; Myocardial infarction; Next-generation sequencing; Transcriptome.
© The Author 2014. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures

References
-
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. - PMC - PubMed
-
- Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. - PubMed
-
- Mercer TR, Mattick JS. Structure and function of long noncoding RNAs in epigenetic regulation. Nat Struct Mol Biol. 2013;20:300–307. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

