NAD+ and sirtuins in aging and disease
- PMID: 24786309
- PMCID: PMC4112140
- DOI: 10.1016/j.tcb.2014.04.002
NAD+ and sirtuins in aging and disease
Abstract
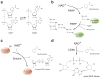
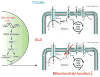
Nicotinamide adenine dinucleotide (NAD(+)) is a classical coenzyme mediating many redox reactions. NAD(+) also plays an important role in the regulation of NAD(+)-consuming enzymes, including sirtuins, poly-ADP-ribose polymerases (PARPs), and CD38/157 ectoenzymes. NAD(+) biosynthesis, particularly mediated by nicotinamide phosphoribosyltransferase (NAMPT), and SIRT1 function together to regulate metabolism and circadian rhythm. NAD(+) levels decline during the aging process and may be an Achilles' heel, causing defects in nuclear and mitochondrial functions and resulting in many age-associated pathologies. Restoring NAD(+) by supplementing NAD(+) intermediates can dramatically ameliorate these age-associated functional defects, counteracting many diseases of aging, including neurodegenerative diseases. Thus, the combination of sirtuin activation and NAD(+) intermediate supplementation may be an effective antiaging intervention, providing hope to aging societies worldwide.
Keywords: NAD(+); nicotinamide mononucleotide; nicotinamide phosphoribosyltransferase; nicotinamide riboside; poly-ADP-ribose polymerases; sirtuins.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures


References
-
- Berger F, et al. The new life of a centenarian: signalling functions of NAD(P) Trends Biochem Sci. 2004;29:111–118. - PubMed
-
- Gellert M, et al. Joining of DNA strands by DNA ligase of E. coli. Cold Spring Harb Symp Quant Biol. 1968;33:21–26. - PubMed
-
- Chambon P, et al. Nicotinamide mononucleotide activation of new DNA-dependent polyadenylic acid synthesizing nuclear enzyme. Biochem Biophys Res Commun. 1963;11:39–43. - PubMed
-
- De Flora A, et al. Autocrine and paracrine calcium signaling by the CD38/NAD+/cyclic ADP-ribose system. Ann N Y Acad Sci. 2004;1028:176–191. - PubMed
-
- Imai S, et al. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

