The c-Abl inhibitor, nilotinib, protects dopaminergic neurons in a preclinical animal model of Parkinson's disease
- PMID: 24786396
- PMCID: PMC4007078
- DOI: 10.1038/srep04874
The c-Abl inhibitor, nilotinib, protects dopaminergic neurons in a preclinical animal model of Parkinson's disease
Abstract
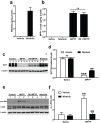
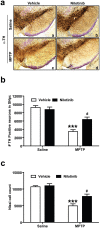
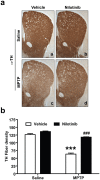
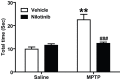
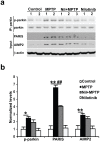
c-Abl is activated in the brain of Parkinson's disease (PD) patients and in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-intoxicated mice where it inhibits parkin through tyrosine phosphorylation leading to the accumulation of parkin substrates, and neuronal cell death. In the present study, we evaluated the in vivo efficacy of nilotinib, a brain penetrant c-Abl inhibitor, in the acute MPTP-induced model of PD. Our results show that administration of nilotinib reduces c-Abl activation and the levels of the parkin substrate, PARIS, resulting in prevention of dopamine (DA) neuron loss and behavioral deficits following MPTP intoxication. On the other hand, we observe no reduction in the tyrosine phosphorylation of parkin and the parkin substrate, AIMP2 suggesting that the protective effect of nilotinib may, in part, be parkin-independent or to the pharmacodynamics properties of nilotinib. This study provides a strong rationale for testing other brain permeable c-Abl inhibitors as potential therapeutic agents for the treatment of PD.
Figures


References
-
- Poewe W., Mahlknecht P. & Jankovic J. Emerging therapies for Parkinson's disease. Curr Opin Neurol 25, 448–459 (2012). - PubMed
-
- Jankovic J. & Poewe W. Therapies in Parkinson's disease. Curr Opin Neurol 25, 433–447 (2012). - PubMed
-
- Dawson T. M. & Dawson V. L. Molecular pathways of neurodegeneration in Parkinson's disease. Science 302, 819–822 (2003). - PubMed
-
- Zhang Y., Dawson V. L. & Dawson T. M. Oxidative stress and genetics in the pathogenesis of Parkinson's disease. Neurobiol Dis 7, 240–250 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

