Rac1 as a potential therapeutic target for chemo-radioresistant head and neck squamous cell carcinomas (HNSCC)
- PMID: 24786604
- PMCID: PMC4037830
- DOI: 10.1038/bjc.2014.221
Rac1 as a potential therapeutic target for chemo-radioresistant head and neck squamous cell carcinomas (HNSCC)
Abstract
Background: In order to improve therapy for HNSCC patients, novel methods to predict and combat local and/or distant tumour relapses are urgently needed. This study has been dedicated to the hypothesis that Rac1, a Rho GTPase, is implicated in HNSCC insensitivity to chemo-radiotherapy resulting in tumour recurrence development.
Methods: Parental and radiation-resistant (IRR) HNSCC cells were used to support this hypothesis. All cells were investigated for their sensitivity to ionising radiation and cisplatin, Rac1 activity, its intracellular expression and subcellular localisation. Additionally, tumour tissues obtained from 60 HNSCC patients showing different therapy response were evaluated for intratumoral Rac1 expression.
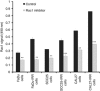
Results: Radiation-resistant IRR cells also revealed resistance to cisplatin accompanied by increased expression, activity and trend towards nuclear translocation of Rac1 protein. Chemical inhibition of Rac1 expression and activity resulted in significant improvement of HNSCC sensitivity to ionising radiation and cisplatin. Preclinical results were confirmed in clinical samples. Although Rac1 was poorly presented in normal mucosa, tumour tissues revealed increased Rac1 expression. The most pronounced Rac1 presence was observed in HNSCC patients with poor early or late responses to chemo-radiotherapy. Tissues taken at recurrence were characterised not only by enhanced Rac1 expression but also increased nuclear Rac1 content.
Conclusions: Increased expression, activity and subcellular localisation of Rac1 could be associated with lower early response rate and higher risk of tumour recurrences in HNSCC patients and warrants further validation in larger independent studies. Inhibition of Rac1 activity can be useful in overcoming treatment resistance and could be proposed for HNSCC patients with primary or secondary chemo-radioresistance.
Figures




References
-
- Ang KK, Zhang QE, Rosenthal DI, Nguyen-Tan P, Sherman EJ, Weber RS, Galvin JM, Schwartz DL, El-Naggar AK, Gillison ML, Jordan R, List MA, Konski AA, Thorstad WL, Trotti A, Beitler JJ, Garden AS, Spanos WJ, Yom SS, Axelrod RS. A randomized phase III trial (RTOG 0522) of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III-IV head and neck squamous cell carcinomas (HNC) J Clin Oncol. 2011;29 (suppl:abstr 5500. - PMC - PubMed
-
- Argiris A, Li Y, Forastiere A. Prognostic factors and long-term survivorship in patients with recurrent or metastatic carcinoma of the head and neck. Cancer. 2004;101:2222–2229. - PubMed
-
- Baghi M, Hambek M, Wagenblast J, May A, Gstoettner W, Knecht R. A phase II trial of docetaxel, cisplatin and 5-fluorouracil in patients with recurrent squamous cell carcinoma of the head and neck (SCCHN) Anticancer Res. 2006;26:585–590. - PubMed
-
- Begg AC. Predicting recurrence after radiotherapy in head and neck cancer. Semin Radiat Oncol. 2012;22:108–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

