Outcomes and statistical power in adult critical care randomized trials
- PMID: 24786714
- PMCID: PMC4226016
- DOI: 10.1164/rccm.201401-0056CP
Outcomes and statistical power in adult critical care randomized trials
Abstract
Rationale: Intensive care unit (ICU)-based randomized clinical trials (RCTs) among adult critically ill patients commonly fail to detect treatment benefits.
Objectives: Appraise the rates of success, outcomes used, statistical power, and design characteristics of published trials.
Methods: One hundred forty-six ICU-based RCTs of diagnostic, therapeutic, or process/systems interventions published from January 2007 to May 2013 in 16 high-impact general or critical care journals were studied.
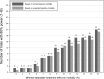
Measurement and main results: Of 146 RCTs, 54 (37%) were positive (i.e., the a priori hypothesis was found to be statistically significant). The most common primary outcomes were mortality (n = 40 trials), infection-related outcomes (n = 33), and ventilation-related outcomes (n = 30), with positive results found in 10, 58, and 43%, respectively. Statistical power was discussed in 135 RCTs (92%); 92 cited a rationale for their power parameters. Twenty trials failed to achieve at least 95% of their reported target sample size, including 11 that were stopped early due to insufficient accrual/logistical issues. Of 34 superiority RCTs comparing mortality between treatment arms, 13 (38%) accrued a sample size large enough to find an absolute mortality reduction of 10% or less. In 22 of these trials the observed control-arm mortality rate differed from the predicted rate by at least 7.5%.
Conclusions: ICU-based RCTs are commonly negative and powered to identify what appear to be unrealistic treatment effects, particularly when using mortality as the primary outcome. Additional concerns include a lack of standardized methods for assessing common outcomes, unclear justifications for statistical power calculations, insufficient patient accrual, and incorrect predictions of baseline event rates.
Keywords: critical care; intensive care; intensive care unit; randomized clinical trial; randomized controlled trial.
Figures



References
-
- Ospina-Tascon GA, Buchele GL, Vincent J-L. Multicenter, randomized, controlled trials evaluating mortality in intensive care: doomed to fail? Crit Care Med. 2008;36:1311–1322. - PubMed
-
- Angus DC, Mira JP, Vincent JL. Improving clinical trials in the critically ill. Crit Care Med. 2010;38:527–532. - PubMed
-
- Annane D. Improving clinical trials in the critically ill: unique challenge–sepsis. Crit Care Med. 2009;37:S117–S128. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

