Quantitative multi-parametric evaluation of centrosome declustering drugs: centrosome amplification, mitotic phenotype, cell cycle and death
- PMID: 24787016
- PMCID: PMC4047924
- DOI: 10.1038/cddis.2014.164
Quantitative multi-parametric evaluation of centrosome declustering drugs: centrosome amplification, mitotic phenotype, cell cycle and death
Abstract
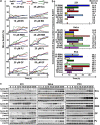
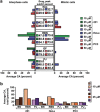
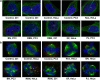
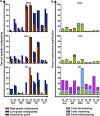
Unlike normal cells, cancer cells contain amplified centrosomes and rely on centrosome clustering mechanisms to form a pseudobipolar spindle that circumvents potentially fatal spindle multipolarity (MP). Centrosome clustering also promotes low-grade chromosome missegregation, which can drive malignant transformation and tumor progression. Putative 'centrosome declustering drugs' represent a cancer cell-specific class of chemotherapeutics that produces a common phenotype of centrosome declustering and spindle MP. However, differences between individual agents in terms of efficacy and phenotypic nuances remain unexplored. Herein, we have developed a conceptual framework for the quantitative evaluation of centrosome declustering drugs by investigating their impact on centrosomes, clustering, spindle polarity, cell cycle arrest, and death in various cancer cell lines at multiple drug concentrations over time. Surprisingly, all centrosome declustering drugs evaluated in our study were also centrosome-amplifying drugs to varying extents. Notably, all declustering drugs induced spindle MP, and the peak extent of MP positively correlated with the induction of hypodiploid DNA-containing cells. Our data suggest acentriolar spindle pole amplification as a hitherto undescribed activity of some declustering drugs, resulting in spindle MP in cells that may not have amplified centrosomes. In general, declustering drugs were more toxic to cancer cell lines than non-transformed ones, with some exceptions. Through a comprehensive description and quantitative analysis of numerous phenotypes induced by declustering drugs, we propose a novel framework for the assessment of putative centrosome declustering drugs and describe cellular characteristics that may enhance susceptibility to them.
Figures


References
-
- Vitale I, Galluzzi L, Castedo M, Kroemer G. Mitotic catastrophe: a mechanism for avoiding genomic instability. Nat Rev Mol Cell Biol. 2011;12:385–392. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

