Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for rasburicase therapy in the context of G6PD deficiency genotype
- PMID: 24787449
- PMCID: PMC4111801
- DOI: 10.1038/clpt.2014.97
Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for rasburicase therapy in the context of G6PD deficiency genotype
Abstract
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is associated with development of acute hemolytic anemia (AHA) induced by a number of drugs. We provide guidance as to which G6PD genotypes are associated with G6PD deficiency in males and females. Rasburicase is contraindicated in G6PD-deficient patients due to the risk of AHA and possibly methemoglobinemia. Unless preemptive genotyping has established a positive diagnosis of G6PD deficiency, quantitative enzyme assay remains the mainstay of screening prior to rasburicase use. The purpose of this article is to help interpret the results of clinical G6PD genotype tests so that they can guide the use of rasburicase. Detailed guidelines on other aspects of the use of rasburicase, including analyses of cost-effectiveness, are beyond the scope of this document. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines are published and updated periodically on https://www.pharmgkb.org/page/cpic to reflect new developments in the field.
Figures
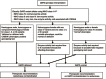
References
-
- Beutler, E. G6PD deficiency. Blood 84, 3613–3636 (1994). - PubMed
-
- Cappellini, M.D. & Fiorelli, G. Glucose‐6‐phosphate dehydrogenase deficiency. Lancet 371, 64–74 (2008). - PubMed
-
- Youngster, I. et al. Medications and glucose‐6‐phosphate dehydrogenase deficiency: an evidence‐based review. Drug Saf. 33, 713–726 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

