Frontal midline theta reflects anxiety and cognitive control: meta-analytic evidence
- PMID: 24787485
- PMCID: PMC4213310
- DOI: 10.1016/j.jphysparis.2014.04.003
Frontal midline theta reflects anxiety and cognitive control: meta-analytic evidence
Abstract
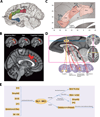
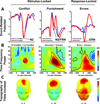
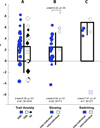
Evidence from imaging and anatomical studies suggests that the midcingulate cortex (MCC) is a dynamic hub lying at the interface of affect and cognition. In particular, this neural system appears to integrate information about conflict and punishment in order to optimize behavior in the face of action-outcome uncertainty. In a series of meta-analyses, we show how recent human electrophysiological research provides compelling evidence that frontal-midline theta signals reflecting MCC activity are moderated by anxiety and predict adaptive behavioral adjustments. These findings underscore the importance of frontal theta activity to a broad spectrum of control operations. We argue that frontal-midline theta provides a neurophysiologically plausible mechanism for optimally adjusting behavior to uncertainty, a hallmark of situations that elicit anxiety and demand cognitive control. These observations compel a new perspective on the mechanisms guiding motivated learning and behavior and provide a framework for understanding the role of the MCC in temperament and psychopathology.
Keywords: Anterior cingulate cortex (ACC); Anxiety; Behavioral inhibition; Cognitive control; Emotion; Error-related negativity (ERN); Feedback-related negativity (FRN); N2; Post-error slowing; Theta.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Amodio DM, Master SL, Yee CM, Taylor SE. Neurocognitive components of the behavioral inhibition and activation systems: implications for theories of self-regulation. Psychophysiology. 2008;45:11–19. - PubMed
-
- Baker TE, Holroyd CB. Dissociated roles of the anterior cingulate cortex in reward and conflict processing as revealed by the feedback error-related negativity and N200. Biol Psychol. 2011 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

