Electronic sculpting of ligand-GPCR subtype selectivity: the case of angiotensin II
- PMID: 24787922
- PMCID: PMC4374176
- DOI: 10.1021/cb500063y
Electronic sculpting of ligand-GPCR subtype selectivity: the case of angiotensin II
Abstract
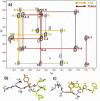
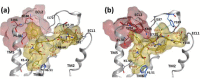
GPCR subtypes possess distinct functional and pharmacological profiles, and thus development of subtype-selective ligands has immense therapeutic potential. This is especially the case for the angiotensin receptor subtypes AT1R and AT2R, where a functional negative control has been described and AT2R activation highlighted as an important cancer drug target. We describe a strategy to fine-tune ligand selectivity for the AT2R/AT1R subtypes through electronic control of ligand aromatic-prolyl interactions. Through this strategy an AT2R high affinity (Ki = 3 nM) agonist analogue that exerted 18,000-fold higher selectivity for AT2R versus AT1R was obtained. We show that this compound is a negative regulator of AT1R signaling since it is able to inhibit MCF-7 breast carcinoma cellular proliferation in the low nanomolar range.
Figures


References
-
- Venkatakrishnan A. J.; Deupi X.; Lebon G.; Tate C. G.; Schertler G. F.; Babu M. M. (2013) Molecular signatures of G-protein-coupled receptors. Nature 494, 185–194. - PubMed
-
- Lagerstrom M. C.; Schioth H. B. (2008) Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discovery 7, 339–357. - PubMed
-
- Tzakos A. G.; Bonvin A. M.; Troganis A.; Cordopatis P.; Amzel M. L.; Gerothanassis I. P.; van Nuland N. A. (2003) On the molecular basis of the recognition of angiotensin II (AII). NMR structure of AII in solution compared with the X-ray structure of AII bound to the mAb Fab131. Eur. J. Biochem. 270, 849–860. - PubMed
-
- Tzakos A. G.; Gerothanassis I. P.; Troganis A. N. (2004) On the structural basis of the hypertensive properties of angiotensin II: a solved mystery or a controversial issue?. Curr. Top Med. Chem. 4, 431–444. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

