Glioma cancer stem cells secrete Gremlin1 to promote their maintenance within the tumor hierarchy
- PMID: 24788093
- PMCID: PMC4035537
- DOI: 10.1101/gad.235515.113
Glioma cancer stem cells secrete Gremlin1 to promote their maintenance within the tumor hierarchy
Abstract
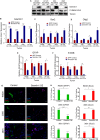
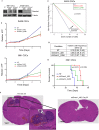
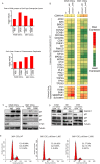
Glioblastomas are the most prevalent and lethal primary brain tumor and are comprised of hierarchies with self-renewing cancer stem cells (CSCs) at the apex. Like neural stem cells (NSCs), CSCs reside in functional niches that provide essential cues to maintain the cellular hierarchy. Bone morphogenetic proteins (BMPs) instruct NSCs to adopt an astrocyte fate and are proposed as anti-CSC therapies to induce differentiation, but, paradoxically, tumors express high levels of BMPs. Here we demonstrate that the BMP antagonist Gremlin1 is specifically expressed by CSCs as protection from endogenous BMPs. Gremlin1 colocalizes with CSCs in vitro and in vivo. Furthermore, Gremlin1 blocks prodifferentiation effects of BMPs, and overexpression of Gremlin1 in non-CSCs decreases their endogenous BMP signaling to promote stem-like features. Consequently, Gremlin1-overexpressing cells display increased growth and tumor formation abilities. Targeting Gremlin1 in CSCs results in impaired growth and self-renewal. Transcriptional profiling demonstrated that Gremlin1 effects were associated with inhibition of p21(WAF1/CIP1), a key CSC signaling node. This study establishes CSC-derived Gremlin1 as a driving force in maintaining glioblastoma tumor proliferation and glioblastoma hierarchies through the modulation of endogenous prodifferentiation signals.
Keywords: BMP antagonists; Gremlin1; bone morphogenetic proteins (BMPs); cancer stem cells; glioblastoma; stem cell hierarchies.
© 2014 Yan et al.; Published by Cold Spring Harbor Laboratory Press.
Figures




Comment in
-
Gremlins sabotage the mechanisms of cancer stem cell differentiation.Cancer Cell. 2014 Jun 16;25(6):716-7. doi: 10.1016/j.ccr.2014.06.002. Cancer Cell. 2014. PMID: 24937457
References
-
- Ades L, Guerci A, Raffoux E, Sanz M, Chevallier P, Lapusan S, Recher C, Thomas X, Rayon C, Castaigne S, et al. 2010. Very long-term outcome of acute promyelocytic leukemia after treatment with all-trans retinoic acid and chemotherapy: the European APL Group experience. Blood 115: 1690–1696 - PubMed
-
- Anido J, Saez-Borderias A, Gonzalez-Junca A, Rodon L, Folch G, Carmona MA, Prieto-Sanchez RM, Barba I, Martinez-Saez E, Prudkin L, et al. 2010. TGF-β receptor inhibitors target the CD44(high)/Id1(high) glioma-initiating cell population in human glioblastoma. Cancer Cell 18: 655–668 - PubMed
-
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN 2006a. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444: 756–760 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
