HIV and HCV activate the inflammasome in monocytes and macrophages via endosomal Toll-like receptors without induction of type 1 interferon
- PMID: 24788318
- PMCID: PMC4006909
- DOI: 10.1371/journal.ppat.1004082
HIV and HCV activate the inflammasome in monocytes and macrophages via endosomal Toll-like receptors without induction of type 1 interferon
Abstract
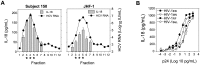
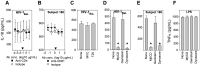
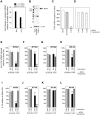
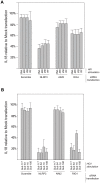
Innate immune sensing of viral infection results in type I interferon (IFN) production and inflammasome activation. Type I IFNs, primarily IFN-α and IFN-β, are produced by all cell types upon virus infection and promote an antiviral state in surrounding cells by inducing the expression of IFN-stimulated genes. Type I IFN production is mediated by Toll-like receptor (TLR) 3 in HCV infected hepatocytes. Type I IFNs are also produced by plasmacytoid dendritic cells (pDC) after sensing of HIV and HCV through TLR7 in the absence of productive pDC infection. Inflammasomes are multi-protein cytosolic complexes that integrate several pathogen-triggered signaling cascades ultimately leading to caspase-1 activation and generation pro-inflammatory cytokines including interleukin (IL)-18 and IL-1β. Here, we demonstrate that HIV and HCV activate the inflammasome, but not Type I IFN production, in monocytes and macrophages in an infection-independent process that requires clathrin-mediated endocytosis and recognition of the virus by distinct endosomal TLRs. Knockdown of each endosomal TLR in primary monocytes by RNA interference reveals that inflammasome activation in these cells results from HIV sensing by TLR8 and HCV recognition by TLR7. Despite its critical role in type I IFN production by pDCs stimulated with HIV, TLR7 is not required for inflammasome activation by HIV. Similarly, HCV activation of the inflammasome in monocytes does not require TLR3 or its downstream signaling adaptor TICAM-1, while this pathway leads to type I IFN in infected hepatocytes. Monocytes and macrophages do not produce type I IFN upon TLR8 or TLR7 sensing of HIV or HCV, respectively. These findings reveal a novel infection-independent mechanism for chronic viral induction of key anti-viral programs and demonstrate distinct TLR utilization by different cell types for activation of the type I IFN vs. inflammasome pathways of inflammation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
HIV-1 infection induces interleukin-1β production via TLR8 protein-dependent and NLRP3 inflammasome mechanisms in human monocytes.J Biol Chem. 2014 Aug 1;289(31):21716-26. doi: 10.1074/jbc.M114.566620. Epub 2014 Jun 17. J Biol Chem. 2014. PMID: 24939850 Free PMC article.
-
HCV RNA Activates APCs via TLR7/TLR8 While Virus Selectively Stimulates Macrophages Without Inducing Antiviral Responses.Sci Rep. 2016 Jul 7;6:29447. doi: 10.1038/srep29447. Sci Rep. 2016. PMID: 27385120 Free PMC article.
-
IFN-γ production by human natural killer cells in response to HCV-infected hepatoma cells is dependent on accessory cells.J Hepatol. 2013 Sep;59(3):442-9. doi: 10.1016/j.jhep.2013.04.022. Epub 2013 May 7. J Hepatol. 2013. PMID: 23665181 Free PMC article.
-
Sex Differences in Primary HIV Infection: Revisiting the Role of TLR7-Driven Type 1 IFN Production by Plasmacytoid Dendritic Cells in Women.Front Immunol. 2021 Aug 27;12:729233. doi: 10.3389/fimmu.2021.729233. eCollection 2021. Front Immunol. 2021. PMID: 34512664 Free PMC article. Review.
-
Innate immunity and HCV.J Hepatol. 2013 Mar;58(3):564-74. doi: 10.1016/j.jhep.2012.10.005. Epub 2012 Oct 11. J Hepatol. 2013. PMID: 23063572 Review.
Cited by
-
Human immunodeficiency virus Type-1 single-stranded RNA activates the NLRP3 inflammasome and impairs autophagic clearance of damaged mitochondria in human microglia.Glia. 2019 May;67(5):802-824. doi: 10.1002/glia.23568. Epub 2018 Dec 24. Glia. 2019. PMID: 30582668 Free PMC article.
-
Deviant Behavior: Tick-Borne Pathogens and Inflammasome Signaling.Vet Sci. 2016 Sep 28;3(4):27. doi: 10.3390/vetsci3040027. Vet Sci. 2016. PMID: 29056735 Free PMC article. Review.
-
Innate immunity against HIV-1 infection.Nat Immunol. 2015 Jun;16(6):554-62. doi: 10.1038/ni.3157. Nat Immunol. 2015. PMID: 25988887 Review.
-
Interplay between Inflammation and Cellular Stress Triggered by Flaviviridae Viruses.Front Microbiol. 2016 Aug 25;7:1233. doi: 10.3389/fmicb.2016.01233. eCollection 2016. Front Microbiol. 2016. PMID: 27610098 Free PMC article. Review.
-
Cytosolic Recognition of Microbes and Pathogens: Inflammasomes in Action.Microbiol Mol Biol Rev. 2018 Sep 12;82(4):e00015-18. doi: 10.1128/MMBR.00015-18. Print 2018 Dec. Microbiol Mol Biol Rev. 2018. PMID: 30209070 Free PMC article. Review.
References
-
- UNAIDS (2011) UNAIDS 2011 World AIDS Day report. http://www.unaids.org/en/resources/publications/2011/name,63525,en.asp
-
- WHO (1997) World Health Organization Hepatitis C: global prevalance. Wkly Epidemiol Rec 341–348. - PubMed
-
- Stetson DB, Medzhitov R (2006) Type I interferons in host defense. Immunity 25: 373–381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

