c-Src drives intestinal regeneration and transformation
- PMID: 24788409
- PMCID: PMC4194090
- DOI: 10.1002/embj.201387454
c-Src drives intestinal regeneration and transformation
Abstract
The non-receptor tyrosine kinase c-Src, hereafter referred to as Src, is overexpressed or activated in multiple human malignancies. There has been much speculation about the functional role of Src in colorectal cancer (CRC), with Src amplification and potential activating mutations in up to 20% of the human tumours, although this has never been addressed due to multiple redundant family members. Here, we have used the adult Drosophila and mouse intestinal epithelium as paradigms to define a role for Src during tissue homeostasis, damage-induced regeneration and hyperplasia. Through genetic gain and loss of function experiments, we demonstrate that Src is necessary and sufficient to drive intestinal stem cell (ISC) proliferation during tissue self-renewal, regeneration and tumourigenesis. Surprisingly, Src plays a non-redundant role in the mouse intestine, which cannot be substituted by the other family kinases Fyn and Yes. Mechanistically, we show that Src drives ISC proliferation through upregulation of EGFR and activation of Ras/MAPK and Stat3 signalling. Therefore, we demonstrate a novel essential role for Src in intestinal stem/progenitor cell proliferation and tumourigenesis initiation in vivo.
Keywords: Apc; Src; intestinal stem cells; regeneration; tumourigenesis.
© 2014 The Authors. Published under the terms of the CC BY NC ND 4.0 license.
Figures
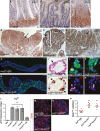
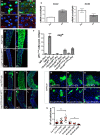
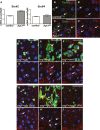
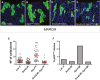
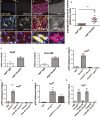
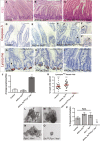
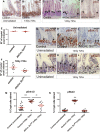
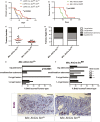
Comment in
-
Epithelial regeneration and cancer: news from the Src front.EMBO J. 2014 Jul 1;33(13):1423-4. doi: 10.15252/embj.201488815. Epub 2014 May 22. EMBO J. 2014. PMID: 24855224 Free PMC article.
References
-
- Ahmed Y, Hayashi S, Levine A, Wieschaus E. Regulation of armadillo by a Drosophila APC inhibits neuronal apoptosis during retinal development. Cell. 1998;93:1171–1182. - PubMed
-
- Andreu P, Colnot S, Godard C, Gad S, Chafey P, Niwa-Kawakita M, Laurent-Puig P, Kahn A, Robine S, Perret C, Romagnolo B. Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development. 2005;132:1443–1451. - PubMed
-
- Ashton GH, Morton JP, Myant K, Phesse TJ, Ridgway RA, Marsh V, Wilkins JA, Athineos D, Muncan V, Kemp R, Neufeld K, Clevers H, Brunton V, Winton DJ, Wang X, Sears RC, Clarke AR, Frame MC, Sansom OJ. Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Dev Cell. 2010;19:259–269. - PMC - PubMed
-
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

