Expression of a humanized viral 2A-mediated lux operon efficiently generates autonomous bioluminescence in human cells
- PMID: 24788811
- PMCID: PMC4008522
- DOI: 10.1371/journal.pone.0096347
Expression of a humanized viral 2A-mediated lux operon efficiently generates autonomous bioluminescence in human cells
Abstract
Background: Expression of autonomous bioluminescence from human cells was previously reported to be impossible, suggesting that all bioluminescent-based mammalian reporter systems must therefore require application of a potentially influential chemical substrate. While this was disproven when the bacterial luciferase (lux) cassette was demonstrated to function in a human cell, its expression required multiple genetic constructs, was functional in only a single cell type, and generated a significantly reduced signal compared to substrate-requiring systems. Here we investigate the use of a humanized, viral 2A-linked lux genetic architecture for the efficient introduction of an autobioluminescent phenotype across a variety of human cell lines.
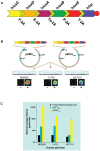
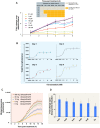
Methodology/principal findings: The lux cassette was codon optimized and assembled into a synthetic human expression operon using viral 2A elements as linker regions. Human kidney, breast cancer, and colorectal cancer cell lines were both transiently and stably transfected with the humanized operon and the resulting autobioluminescent phenotype was evaluated using common imaging instrumentation. Autobioluminescent cells were screened for cytotoxic effects resulting from lux expression and their utility as bioreporters was evaluated through the demonstration of repeated monitoring of single populations over a prolonged period using both a modified E-SCREEN assay for estrogen detection and a classical cytotoxic compound detection assay for the antibiotic Zeocin. Furthermore, the use of self-directed bioluminescent initiation in response to target detection was assessed to determine its amenability towards deployment as fully autonomous sensors. In all cases, bioluminescent measurements were supported with traditional genetic and transcriptomic evaluations.
Conclusions/significance: Our results demonstrate that the viral 2A-linked, humanized lux genetic architecture successfully produced autobioluminescent phenotypes in all cell lines tested without the induction of cytotoxicity. This autobioluminescent phenotype allowed for repeated interrogation of populations and self-directed control of bioluminescent activation with detection limits and EC50 values similar to traditional reporter systems, making the autobioluminescent cells amenable to automated monitoring and significantly reducing the time and cost required to perform bioluminescent workflows.
Conflict of interest statement
Figures
References
-
- Constance J (2010) Molecular imaging markets. Kalorama Information Market Intelligence Report. New York, NY.
-
- de Wet J, Wood K, Helinski D, DeLuca M (1986) Cloning firefly luciferase. Methods in Enzymology 133: 3–14. - PubMed
-
- Promega (2009) Technical Manual: pGL4 Luciferase. Promega Corporation.
-
- Verhaegen M, Christopoulos T (2002) Recombinant Gaussia luciferase. Overexpression, purification, and analytical application of a bioluminescent reporter for DNA hybridization. Anal Chem 74: 4378–4385. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

