Ester carbonyl vibration as a sensitive probe of protein local electric field
- PMID: 24788907
- PMCID: PMC4104746
- DOI: 10.1002/anie.201402011
Ester carbonyl vibration as a sensitive probe of protein local electric field
Abstract
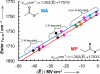
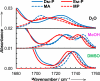
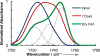
The ability to quantify the local electrostatic environment of proteins and protein/peptide assemblies is key to gaining a microscopic understanding of many biological interactions and processes. Herein, we show that the ester carbonyl stretching vibration of two non-natural amino acids, L-aspartic acid 4-methyl ester and L-glutamic acid 5-methyl ester, is a convenient and sensitive probe in this regard, since its frequency correlates linearly with the local electrostatic field for both hydrogen-bonding and non-hydrogen-bonding environments. We expect that the resultant frequency-electric-field map will find use in various applications. Furthermore, we show that, when situated in a non-hydrogen-bonding environment, this probe can also be used to measure the local dielectric constant (ε). For example, its application to amyloid fibrils formed by Aβ(16-22) revealed that the interior of such β-sheet assemblies has an ε value of approximately 5.6.
Keywords: IR spectroscopy; carbonyl groups; hydrogen bonds; protein electrostatics; vibrational probes.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

References
-
- Boxer SG. J. Phys. Chem. B. 2009;113:2972–2983. - PubMed
-
- Kim H, Cho M. Chem. Rev. 2013:5817–5847. - PubMed
-
- Volk M, Kholodenko Y, Lu HSM, Gooding EA, DeGrado WF, Hochstrasser RM. J. Phys. Chem. B. 1997;101:8607–8616.
- Schkolnik G, Utesch T, Salewski J, Tenger K, Millo D, Kranich A, Zebger I, Schulz C, Zimanyi L, Rakhely G, Mroginski MA, Hildebrandt P. Chem. Commun. 2012;48:70–72. - PubMed
- Chung JK, Thielges MC, Fayer MD. J. Am. Chem. Soc. 2012;134:12118–12124. - PMC - PubMed
- Walker DM, Hayes EC, Webb LJ. Phys. Chem. Chem. Phys. 2013;15:12241–12252. - PubMed
-
- Dyer RB, Gai F, Woodruff WH. Acc. Chem. Res. 1998;31:709–716.
- Woutersen S, Pfister R, Hamm P, Mu YG, Kosov DS, Stock G. J. Chem. Phys. 2002;117:6833–6840.
- Demirdoven N, Cheatum CM, Chung HS, Khalil M, Knoester J, Tokmakoff A. J. Am. Chem. Soc. 2004;126:7981–7990. - PubMed
- Waegele MM, Culik RM, Gai F. J. Phys. Chem. Lett. 2011;2:2598–2609. - PMC - PubMed
- Serrano AL, Waegele MM, Gai F. Protein Sci. 2012;21:157–170. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

