Disrupted bone remodeling leads to cochlear overgrowth and hearing loss in a mouse model of fibrous dysplasia
- PMID: 24788917
- PMCID: PMC4006800
- DOI: 10.1371/journal.pone.0094989
Disrupted bone remodeling leads to cochlear overgrowth and hearing loss in a mouse model of fibrous dysplasia
Abstract
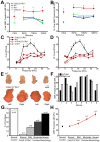
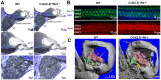
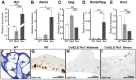
Normal hearing requires exquisite cooperation between bony and sensorineural structures within the cochlea. For example, the inner ear secretes proteins such as osteoprotegrin (OPG) that can prevent cochlear bone remodeling. Accordingly, diseases that affect bone regulation can also result in hearing loss. Patients with fibrous dysplasia develop trabecular bone overgrowth resulting in hearing loss if the lesions affect the temporal bones. Unfortunately, the mechanisms responsible for this hearing loss, which could be sensorineural and/or conductive, remain unclear. In this study, we used a unique transgenic mouse model of increased Gs G-protein coupled receptor (GPCR) signaling induced by expression of an engineered receptor, Rs1, in osteoblastic cells. These ColI(2.3)+/Rs1+ mice showed dramatic bone lesions that histologically and radiologically resembled fibrous dysplasia. We found that ColI(2.3)+/Rs1+ mice showed progressive and severe conductive hearing loss. Ossicular chain impingement increased with the size and number of dysplastic lesions. While sensorineural structures were unaffected, ColI(2.3)+/Rs1+ cochleae had abnormally high osteoclast activity, together with elevated tartrate resistant acid phosphatase (TRAP) activity and receptor activator of nuclear factor kappa-B ligand (Rankl) mRNA expression. ColI(2.3)+/Rs1+ cochleae also showed decreased expression of Sclerostin (Sost), an antagonist of the Wnt signaling pathway that normally increases bone formation. The osteocyte canalicular networks of ColI(2.3)+/Rs1+ cochleae were disrupted and showed abnormal osteocyte morphology. The osteocytes in the ColI(2.3)+/Rs1+ cochleae showed increased expression of matrix metalloproteinase 13 (MMP-13) and TRAP, both of which can support osteocyte-mediated peri-lacunar remodeling. Thus, while the ossicular chain impingement is sufficient to account for the progressive hearing loss in fibrous dysplasia, the deregulation of bone remodeling extends to the cochlea as well. Our findings suggest that factors regulating bone remodeling, including peri-lacunar remodeling by osteocytes, may be useful targets for treating the bony overgrowths and hearing changes of fibrous dysplasia and other bony pathologies.
Conflict of interest statement
Figures


Similar articles
-
Parallel mechanisms suppress cochlear bone remodeling to protect hearing.Bone. 2016 Aug;89:7-15. doi: 10.1016/j.bone.2016.04.010. Epub 2016 Apr 13. Bone. 2016. PMID: 27085457 Free PMC article.
-
Association of Hearing Loss and Otologic Outcomes With Fibrous Dysplasia.JAMA Otolaryngol Head Neck Surg. 2018 Feb 1;144(2):102-107. doi: 10.1001/jamaoto.2017.2407. JAMA Otolaryngol Head Neck Surg. 2018. PMID: 29192304 Free PMC article.
-
Matrix metalloproteinase-13 is required for osteocytic perilacunar remodeling and maintains bone fracture resistance.J Bone Miner Res. 2012 Sep;27(9):1936-50. doi: 10.1002/jbmr.1646. J Bone Miner Res. 2012. PMID: 22549931 Free PMC article.
-
Fibrous dysplasia of the temporal bone: ten new cases demonstrating the spectrum of otologic sequelae.Am J Otol. 1995 Jul;16(4):408-19. Am J Otol. 1995. PMID: 8588639 Review.
-
Osteocyte control of bone remodeling: is sclerostin a key molecular coordinator of the balanced bone resorption-formation cycles?Osteoporos Int. 2014 Dec;25(12):2685-700. doi: 10.1007/s00198-014-2808-0. Epub 2014 Jul 17. Osteoporos Int. 2014. PMID: 25030653 Review.
Cited by
-
Characterization of hearing-impairment in Generalized Arterial Calcification of Infancy (GACI).Orphanet J Rare Dis. 2022 Jul 19;17(1):273. doi: 10.1186/s13023-022-02410-w. Orphanet J Rare Dis. 2022. PMID: 35854274 Free PMC article.
-
Hypermineralization of Hearing-Related Bones by a Specific Osteoblast Subtype.J Bone Miner Res. 2021 Aug;36(8):1535-1547. doi: 10.1002/jbmr.4320. Epub 2021 May 14. J Bone Miner Res. 2021. PMID: 33905562 Free PMC article.
-
Parallel mechanisms suppress cochlear bone remodeling to protect hearing.Bone. 2016 Aug;89:7-15. doi: 10.1016/j.bone.2016.04.010. Epub 2016 Apr 13. Bone. 2016. PMID: 27085457 Free PMC article.
-
Evolutionary Basis of High-Frequency Hearing in the Cochleae of Echolocators Revealed by Comparative Genomics.Genome Biol Evol. 2020 Jan 1;12(1):3740-3753. doi: 10.1093/gbe/evz250. Genome Biol Evol. 2020. PMID: 31730196 Free PMC article.
-
Association of Hearing Loss and Otologic Outcomes With Fibrous Dysplasia.JAMA Otolaryngol Head Neck Surg. 2018 Feb 1;144(2):102-107. doi: 10.1001/jamaoto.2017.2407. JAMA Otolaryngol Head Neck Surg. 2018. PMID: 29192304 Free PMC article.
References
-
- Lyford-Pike S, Hoover-Fong J, Tunkel DE (2012) Otolaryngologic manifestations of skeletal dysplasias in children. Otolaryngologic clinics of North America 45: 579–598, vii. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

