Anti-vascular endothelial growth factor for macular oedema secondary to central retinal vein occlusion
- PMID: 24788977
- PMCID: PMC4292843
- DOI: 10.1002/14651858.CD007325.pub3
Anti-vascular endothelial growth factor for macular oedema secondary to central retinal vein occlusion
Abstract
Background: Central retinal vein occlusion (CRVO) is a relatively common retinal vascular disorder in which macular oedema may develop, with a consequent reduction in visual acuity. Until recently there has been no treatment of proven benefit, but growing evidence supports the use of anti-vascular endothelial growth factor (anti-VEGF) agents.
Objectives: To investigate the effectiveness and safety of anti-VEGF therapies for the treatment of macular oedema secondary to CRVO.
Search methods: We searched CENTRAL (which contains the Cochrane Central Register of Controlled Trials (CENTRAL) and the Cochrane Eyes and Vision Group Trials Register) (The Cochrane Library 2013, Issue 10), Ovid MEDLINE (January 1950 to October 2013), EMBASE (January 1980 to October 2013), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to October 2013), Cumulative Index to Nursing and Allied Health Literature (CINAHL) (January 1937 to October 2013), OpenGrey, OpenSIGLE (January 1950 to October 2013), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com), ClinicalTrials.gov (www.clinicaltrials.gov), the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en) and Web of Science Conference Proceedings Citation Index-Science (CPCI-S). There were no language or date restrictions in the electronic search for trials. The electronic databases and clinical trials registers were last searched on 29th October 2013.
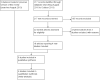
Selection criteria: We considered randomised controlled trials (RCTs) that compared intravitreal anti-VEGF agents of any dose or duration to sham injection or no treatment. We focused on studies that included individuals of any age or gender and a minimum of six months follow-up.
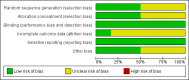
Data collection and analysis: Two review authors independently assessed trial quality and extracted data. The primary outcome was the proportion of participants with a gain in best-corrected visual acuity (BCVA) from baseline of greater than or equal to 15 letters (3 lines) on the Early Treatment of Diabetic Retinopathy Study (ETDRS) chart. Secondary outcomes included the proportion of participants with a loss of 15 letters or more of BCVA, the mean change from baseline BCVA, the mean change in central retinal thickness (CRT), the number and type of complications or adverse outcomes, and the number of additional interventions administered. Where available, we also presented quality of life and economic data.
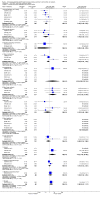
Main results: We found six RCTs that met the inclusion criteria after independent and duplicate review of the search results. These RCTs included 937 participants and compared outcomes at six months to sham injection for four anti-VEGF agents: aflibercept (VEGF Trap-Eye, Eylea), bevacizumab (Avastin), pegaptanib sodium (Macugen) and ranibizumab (Lucentis). Three trials were conducted in Norway, Sweden and the USA, and three trials were multicentre, one including centres in the USA, Canada, India, Israel, Argentina and Columbia, a second including centres in the USA, Australia, France, Germany, Israel, and Spain, and a third including centres in Austria, France, Germany, Hungary, Italy, Latvia, Australia, Japan, Singapore and South Korea. We performed meta-analysis on three key visual outcomes, using data from up to six trials. High-quality evidence from six trials revealed that participants receiving intravitreal anti-VEGF treatment were 2.71 times more likely to gain at least 15 letters of visual acuity at six months compared to participants treated with sham injections (risk ratio (RR) 2.71; 95% confidence intervals (CI) 2.10 to 3.49). High-quality evidence from five trials suggested anti-VEGF treatment was associated with an 80% lower risk of losing at least 15 letters of visual acuity at six months compared to sham injection (RR 0.20; 95% CI 0.12 to 0.34). Moderate-quality evidence from three trials (481 participants) revealed that the mean reduction from baseline to six months in central retinal thickness was 267.4 µm (95% CI 211.4 µm to 323.4 µm) greater in participants treated with anti-VEGF than in participants treated with sham. The meta-analyses demonstrate that treatment with anti-VEGF is associated with a clinically meaningful gain in vision at six months. One trial demonstrated sustained benefit at 12 months compared to sham. No significant ocular or systemic safety concerns were identified in this time period.
Authors' conclusions: Compared to no treatment, repeated intravitreal injection of anti-VEGF agents in eyes with CRVO macular oedema improved visual outcomes at six months. All agents were relatively well tolerated with a low incidence of adverse effects in the short term. Future trials should address the relative efficacy and safety of the anti-VEGF agents and other treatments, including intravitreal corticosteroids, for longer-term outcomes.
Conflict of interest statement
All authors have no known conflicts of interest.
Figures










Update of
-
Anti-vascular endothelial growth factor for macular edema secondary to central retinal vein occlusion.Cochrane Database Syst Rev. 2010 Oct 6;(10):CD007325. doi: 10.1002/14651858.CD007325.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2014 May 01;(5):CD007325. doi: 10.1002/14651858.CD007325.pub3. PMID: 20927757 Free PMC article. Updated.
References
References to studies included in this review
Copernicus 2012 {published data only}
-
- Boyer D, Heier J, Brown DM, Clark WL, Berliner AJ, Groetzbach G, et al. Vascular endothelial growth factor Trap‐Eye for macular edema secondary to central retinal vein occlusion: six‐month results of the phase 3 COPERNICUS study. Ophthalmology 2012;119(5):1024‐32. - PubMed
-
- Brown DM, Heier JS, Clark WL, Boyer DS, Vitti R, Berliner AJ, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1‐year results from the phase 3 COPERNICUS study. American Journal of Ophthalmology 2013;155(3):429‐37. - PubMed
CRUISE 2010 {published data only}
-
- Bhisitkul RB, Campochiaro PA, Shapiro H, Rubio RG. Predictive value in retinal vein occlusions of early versus late or incomplete ranibizumab response defined by optical coherence tomography. Ophthalmology 2013;120(5):1057‐63. - PubMed
-
- Brown DM, Campochiaro PA, Singh RP, Li Z, Gray S, Saroj N, et al. Ranibizumab for macular edema following central retinal vein occlusion: six‐month primary end point results of a phase III study. Ophthalmology 2010;117(6):1124‐33. - PubMed
-
- Campochiaro PA, Bhisitkul RB, Shapiro H, Rubio RG. Vascular endothelial growth factor promotes progressive retinal nonperfusion in patients with retinal vein occlusion. Ophthalmology 2013;120(4):795‐802. - PubMed
-
- Campochiaro PA, Brown DM, Awh CC, Lee SY, Gray S, Saroj N, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve‐month outcomes of a phase III study. Ophthalmology 2011;118:2041‐9. - PubMed
-
- Heier JS, Campochiaro PA, Yau L, Li Z, Saroj N, Rubio RG, Lai P. Ranibizumab for macular edema due to retinal vein occlusions: long‐term follow‐up in the HORIZON trial. Ophthalmology 2012;119:802‐9. - PubMed
Epstein 2012 {published data only}
-
- Epstein DL, Algvere PV, Wendt G, Seregard S, Kvanta A. Benefit from bevacizumab for macular edema in central retinal vein occlusion: twelve‐month results from a prospective, randomized study. Ophthalmology 2012;119(12):2587‐91. - PubMed
-
- Epstein DL, Algvere PV, Wendt G, Seregard S, Kvanta A. Bevacizumab for macular edema in central retinal vein occlusion: a prospective, randomized, double‐masked clinical study. Ophthalmology 2012;119(6):1184‐9. - PubMed
GALILEO 2013 {published data only (unpublished sought but not used)}
-
- Holz FG, Roider J, Ogura Y, Korobelnik JF, Simader C, Groetzbach G, et al. VEGF Trap‐Eye for macular oedema secondary to central retinal vein occlusion: 6‐month results of the phase III GALILEO study. British Journal of Ophthalmology 2013;97(3):278‐84. - PubMed
-
- Korobelnik JF, Holz FG, Roider J, Ogura Y, Simader C, Schmidt‐Erfurth U, for the GALILEO Study Group. Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: one‐year results of the Phase 3 GALILEO Study. Ophthalmology 2014;121(1):202‐8. - PubMed
ROCC 2010 {published data only}
-
- Kinge B, Stordahl PB, Forsaa V, Fossen K, Haugstad M, Helgesen OH, et al. Efficacy of ranibizumab in patients with macular edema secondary to central retinal vein occlusion: results from the sham‐controlled ROCC Study. American Journal of Ophthalmology 2010;150(3):310‐4. - PubMed
Wroblewski 2009 {published data only}
-
- Ciulla TA. Treatment of macular edema following central retinal vein occlusion with pegaptanib sodium (Macugen): a one‐year study. American Academy of Ophthalmology 2007. Conference abstract 199.
-
- Csaky KG. Pegaptanib (Macugen) for macular edema in central retinal vein occlusion: early OCT results and effect of therapy reinitiation. American Academy of Ophthamology 2007. Conference abstract 269.
-
- Patel SS. Pegaptanib sodium for the treatment of macular edema following central retinal vein occlusion (CRVO): anatomical outcomes. Investigative Ophthalmology and Visual Science 2007; Vol. 48:ARVO E‐abstract 311.
-
- Wells JA. Pegaptanib sodium for treatment of macular edema secondary to central retinal vein occlusion. Investigative Ophthalmology and Visual Science 2006; Vol. 47:ARVO E‐abstract 4279.
-
- Wells JA. Safety and efficacy of pegaptanib sodium in treating macular edema secondary to central retinal vein occlusion. American Academy of Ophthalmology 2006. Conference abstract 288.
References to studies excluded from this review
Byeon 2009 {published data only}
-
- Byeon SH, Kwon OW, Song JH, Kim SE, Park YS. Prolongation of activity of single intravitreal bevacizumab by adjuvant topical aqueous depressant (Timolol‐Dorzolamide). Graefes Archive for Clinical and Experimental Ophthalmology 2009;247(1):35‐42. - PubMed
Campochiaro 2008 {published data only}
-
- Campochiaro PA, Hafiz G, Shah SM, Nguyen QD, Ying H, Do DV, et al. Ranibizumab for macular edema due to retinal vein occlusions: implication of VEGF as a critical stimulator. Molecular Therapy 2008;16(4):791‐9. - PubMed
-
- Campochiaro PA, Shah SM, Hafiz G, Quinlan E, Zimmer‐Gller I, Nguyen QD, et al. Ranibizumab for macular edema due to retinal vein occlusions. Investigative Ophthalmology and Visual Science 2007; Vol. 48. ARVO E‐abstract 1545.
Ding 2011 {published data only}
-
- Ding X, Li J, Hu X, Yu S, Pan J, Tang S. Prospective study of intravitreal triamcinolone acetonide versus bevacizumab for macular edema secondary to central retinal vein occlusion. Retina (Philadelphia, Pa.) 2011;31(5):838‐45. - PubMed
Wang 2011 {published data only}
References to studies awaiting assessment
Habibabadi 2008 {unpublished data only}
-
- Habibabadi H, Moradian S, Piri N, Esfahani M, Aalami Z, Lashay A, et al. Intravitreal bevacizumab vs combination of bevacizumab and triamcinolone vs sham treatment in central retinal vein occlusion. American Academy of Ophthalmology 2008. Conference abstract 271.
-
- Habibabadi HF, Moradian S, Piri N, Esfahani MR, Aalami Z, Karkhaneh R, et al. Intravitreal bevacizumab vs combination of bevacizumab and triamcinolone vs sham treatment for central retinal vein occlusion. American Academy of Ophthalmology 2007. Conference abstract 273.
Additional references
Adamis 1996
-
- Adamis AP, Shima DT, Tolentino MJ, Gragoudas ES, Ferrara N, Folkman J, et al. Inhibition of vascular endothelial growth factor prevents retinal ischemia‐associated iris neovascularization in a nonhuman primate. Archives of Ophthalmology 1996;114(1):66‐71. - PubMed
Aggermann 2012
-
- Aggermann T, Brunner S, Krebs I, Haas P, Womastek I, Brannath W, et al. ROVO Study Group. A prospective, randomised, multicenter trial for surgical treatment of central retinal vein occlusion: results of the Radial Optic Neurotomy for Central Vein Occlusion (ROVO) study group. Graefes Archive for Clinical and Experimental Ophthalmology 2013;251(4):1065‐72. - PubMed
Aiello 1995
-
- Aiello LP, Pierce EA, Foley ED, Takagi H, Chen HRL, Ferrara N, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF‐receptor chimeric proteins. Proceedings of the National Academy of Sciences 1995;92(23):10457‐61. - PMC - PubMed
Arevalo 2008
-
- Arevalo JF, Garcia RA, Wu L, Rodriguez FJ, Dalma‐Weiszhausz J, Quiroz‐Mercado H, et al. Radial optic neurotomy for central retinal vein occlusion: results of the Pan‐American Collaborative Retina Study Group (PACORES). Retina 2008;28(8):1044‐52. - PubMed
Bakri 2007a
-
- Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzat MK, Singh RJ. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology 2007;114(12):2179‐82. - PubMed
Bakri 2007b
-
- Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology 2007;114(5):855‐9. - PubMed
Beck 2007
-
- Beck RW, Maguire MG, Bressler NM, Glassman AR, Lindblad AS, Ferris FL. Visual acuity as an outcome measure in clinical trials of retinal disease. Ophthalmology 2007;114(10):1804‐9. - PubMed
Bertelsen 2013
-
- Bertelsen M, Linneberg A, Christoffersen N, Vorum H, Gade E, Larsen M. Mortality in patients with central retinal vein occlusion. Ophthalmology 2014;121(3):637‐42. - PubMed
Boyd 2002
-
- Boyd SR, Zachary I, Chakravarthy U, Allen GJ, Wisdom GB, Cree IA, et al. Correlation of increased vascular endothelial growth factor with neovascularization and permeability in ischemic central vein occlusion. Archives of Ophthalmology 2002;120(12):1644‐50. - PubMed
Campochiaro 2009
-
- Campochiaro PA, Choy DF, Do DV, Gulnar H, Shah SM, Nguyen QD, et al. Monitoring ocular drug therapy by analysis of aqueous samples. Ophthalmology 2009;116(11):2158‐64. - PubMed
Campochiaro 2010
-
- Campochiaro PA, Hafiz G, Channa R, Shah SM, Nguyen QD, Ying H, et al. Antagonism of vascular endothelial growth factor for macular edema caused by retinal vein occlusions: two‐year outcomes. Ophthalmology 2010;117(12):2387‐94. - PubMed
Catier 2005
-
- Catier A, Tadayoni R, Paques M, Erginay A, Haouchine B, Gaudric A, et al. Characterization of macular edema from various etiologies by optical coherence tomography. American Journal of Ophthalmology 2005;140(2):200‐6. - PubMed
Chan 2011
Clarkson 1994
CVOS Group 1995
-
- CVOS Group. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. The Central Vein Occlusion Study Group M report. Ophthalmology 1995;102(10):1425‐33. - PubMed
CVOS Group 1997
-
- CVOS Group. Natural history and clinical management of central retinal vein occlusion. The Central Vein Occlusion Study Group. Archives of Ophthalmology 1997;115(10):1275. - PubMed
DeCroos 2009
-
- DeCroos FC, Shuler RK Jr, Stinnett S, Fekrat S. Pars plana vitrectomy, internal limiting membrane peeling, and panretinal endophotocoagulation for macular edema secondary to central retinal vein occlusion. American Journal of Ophthalmology 2009;147(4):627‐33. - PubMed
Drechsler 2012
-
- Drechsler F, Koferl P, Hollborn M, Wiedemann P, Bringmann A, Kohen L, Rehak M. Effect of intravitreal anti‐vascular endothelial growth factor treatment on the retinal gene expression in acute experimental central retinal vein occlusion. Ophthalmic Research 2012;47(3):157‐62. - PubMed
Economides 2003
-
- Economides AN, Carpenter LR, Rudge JS, Wong V, Koehler‐Stec EM, Hartnett C, et al. Cytokine traps: multi‐component, high‐affinity blockers of cytokine action. Nature Medicine 2003;9(1):47‐52. - PubMed
Edelman 2005
-
- Edelman JL, Lutz D, Castro MR. Corticosteroids inhibit VEGF‐induced vascular leakage in a rabbit model of blood‐retinal and blood‐aqueous barrier breakdown. Experimental Eye Research 2005;80(2):249‐58. - PubMed
Everett 2006
-
- Everett AI, Yang SS. Venous occlusive disease: the latest in current management. Retina 2006;26(6):S63‐4. - PubMed
Eyetech 2008
-
- Eyetech, Inc. Macugen ‐ Pegatanib sodium injection. www.macugen.com/macugenUSPI.pdf 2008.
Feng 2013
Ferrara 2006
-
- Ferrara N, Damico L, Shams N, Lowman H, Kim R. Development of ranibizumab, an anti‐vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age‐related macular degeneration. Retina (Philadelphia, Pa.) 2006;26(8):859‐70. - PubMed
Funk 2009
-
- Funk M, Kriechbaum K, Prager F, Benesch T, Georgopoulos M, Zlabinger GJ, et al. Intraocular concentrations of growth factors and cytokines in retinal vein occlusion and the effect of therapy with bevacizumab. Investigative Ophthalmology & Visual Science 2009;50(3):1025‐32. - PubMed
Genentech 2008
-
- Genentech, Inc. Lucentis: Full prescribing information. www.gene.com/gene/products/information/pdf/lucentis‐prescribing.pdf 2008.
Genentech 2009
-
- Genentech, Inc. Avastin: Full prescribing information. www.gene.com/gene/products/information/pdf/avastin‐prescribing.pdf 2009.
Genentech/Roche 2009a
-
- Genentech/Roche. Lucentis ‐ Ranibizumab injection: Product Information Home. www.gene.com/gene/products/information/tgr/lucentis/ 2009.
Genentech/Roche 2009b
-
- Genentech/Roche. Avastin: Product Information Home. www.gene.com/gene/products/information/oncology/avastin/ 2009.
Gewaily 2009
Glanville 2006
Gragoudas 2004
-
- Gragoudas ES, Adamis AP, Cunningham ET, Feinsod Jr M, Guyer DR. Pegaptanib for neovascular age‐related macular degeneration. New England Journal of Medicine 2004;351(27):2805‐16. - PubMed
Gregori 2010
-
- Gregori NZ, Feuer W, Rosenfeld PJ. Novel method for analyzing Snellen visual acuity measurements. Retina 2010;30(7):1046‐50. - PubMed
Guex‐Crosier 1999
-
- Guex‐Crosier Y. The pathogenesis and clinical presentation of macular edema in inflammatory diseases. Documenta Ophthalmologica 1999;97(3‐4):297‐309. - PubMed
Hasselbach 2007
-
- Hasselbach HC, Ruefer F, Feltgen N, Schneider U, Bopp S, Hansen LL, et al. Treatment of central retinal vein occlusion by radial optic neurotomy in 107 cases. Graefes Archive for Clinical and Experimental Ophthalmology 2007;245(8):1145‐56. - PubMed
Hayreh 1983
-
- Hayreh SS. Classification of central retinal vein occlusion. Ophthalmology 1983;90(5):458‐74. - PubMed
Hayreh 1990a
-
- Hayreh SS, Klugman MR, Beri M, Kimura AE, Podhajsky P. Differentiation of ischemic from non‐ischemic central retinal vein occlusion during the early acute phase. Graefes Archive for Clinical and Experimental Ophthalmology 1990;228(3):201‐17. - PubMed
Hayreh 1990b
-
- Hayreh SS, Klugman MR, Podhajsky P, Servais GE, Perkins ES. Argon laser panretinal photocoagulation in ischemic central retinal vein occlusion. A 10‐year prospective study. Graefes Archive for Clinical and Experimental Ophthalmology 1990;228(4):281‐96. - PubMed
Hayreh 1994
-
- Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrence and demographic characteristics. American Journal of Ophthalmology 1994;117(4):429‐41. - PubMed
Hayreh 2003
-
- Hayreh SS. Management of central retinal vein occlusion. Ophthalmologica 2003;217(3):167‐88. - PubMed
Hayreh 2011
Hee 1995
-
- Hee MR, Puliafito CA, Wong C, Duker JS, Reichel E, Rutledge B, et al. Quantitative assessment of macular edema with optical coherence tomography. Archives of Ophthalmology 1995;113(8):1019‐29. - PubMed
Heier 2012
-
- Heier JS, Campochiaro PA, Yau L, Li Z, Saroj N, Rubio RG, et al. Ranibizumab for macular edema due to retinal vein occlusions: long‐term follow‐up in the HORIZON trial. Ophthalmology 2012;119(4):802‐9. - PubMed
Higgins 2011
-
- Higgins JPT, Green S. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Homsi 2007
-
- Homsi J, Daud AI. Spectrum of activity and mechanism of action of VEGF/PDGF inhibitors. Cancer Control 2007;14(3):285‐94. - PubMed
Krohne 2008
-
- Krohne TU, Eter N, Holz FG, Meyer CH. Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. American Journal of Ophthalmology 2008;146(4):508‐12. - PubMed
Los 2007
-
- Los M, Roodhart JM, Voest EE. Target practice: lessons from phase III trials with bevacizumab and vatalanib in the treatment of advanced colorectal cancer. Oncologist 2007;12(4):443‐50. - PubMed
Lynch 2007
-
- Lynch SS, Cheng CM. Bevacizumab for neovascular ocular diseases. Annals of Pharmacotherapy 2007;41(4):614‐25. - PubMed
McAllister 2010
-
- McAllister IL, Gillies ME, Smithies LA, Rochtchina E, Harper CA, Daniell MD, et al. The Central Retinal Vein Bypass Study: a trial of laser‐induced chorioretinal venous anastomosis for central retinal vein occlusion. Ophthalmology 2010;117(5):954‐65. - PubMed
McIntosh 2010
-
- McIntosh RL, Rogers SL, Lim L, Cheung N, Wang JJ, Mitchell P, et al. Natural history of central retinal vein occlusion: an evidence‐based systematic review. Ophthalmology 2010;117(6):1113‐23. - PubMed
Meyer 2011
-
- Meyer CH, Michels S, Rodrigues EB, Hager A, Mennel S, Schmidt JC, et al. Incidence of rhegmatogenous retinal detachments after intravitreal antivascular endothelial factor injections. Acta Ophthalmologica 2011;89(1):70‐5. - PubMed
Nishijima 2007
Opremcak 2006
-
- Opremcak EM, Rehmar AJ, Ridenour CD, Kurz DE. Radial optic neurotomy for central retinal vein occlusion: 117 consecutive cases. Retina 2006;26(3):297‐305. - PubMed
Ozurdex GENEVA 2010
-
- Haller JA, Bandello F, Belfort R, Blumenkranz MS, Gillies M, Heier J, et al. The OZURDEX GENEVA Study Group. Randomized, sham‐controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology 2010;117(6):1134‐46. - PubMed
Ozurdex GENEVA 2011
-
- Haller JA, Bandello F, Belfort R Jr, Blumenkranz MS, Gillies M, Heier J, et al. The OZURDEX GENEVA Study Group. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve‐month study results. Ophthalmology 2011;118(12):2453‐60. - PubMed
Park 2007
-
- Park CH, Scott AW, Fekrat S. Effect of oral pentoxifylline on cystoid macular edema associated with central retinal vein occlusion. Retina 2007;27(8):1020‐5. - PubMed
Park 2010
-
- Park DH, Kim IT. Long‐term effects of vitrectomy and internal limiting membrane peeling for macular edema secondary to central retinal vein occlusion and hemiretinal vein occlusion. Retina 2010;30(1):117‐24. - PubMed
Pe'er 1995
-
- Pe'er J, Shweiki D, Itin A, Hemo I, Gnessin H, Keshet E. Hypoxia‐induced expression of vascular endothelial growth factor by retinal cells is a common factor in neovascularizing ocular diseases. Laboratory Investigation 1995;72(6):638‐45. - PubMed
Pe'er 1998
-
- Pe'er J, Folberg R, Itin A, Gnessin H, Hemo I, Keshet E. Vascular endothelial growth factor upregulation in human central retinal vein occlusion. Ophthalmology 1998;105(3):412‐6. - PubMed
Prasad 2010
-
- Prasad PS, Oliver SC, Coffee RE, Hubschman JP, Schwartz SD. Ultra wide‐field angiographic characteristics of branch retinal and hemicentral retinal vein occlusion. Ophthalmology 2010;117(4):780‐4. - PubMed
Prisco 2002
-
- Prisco D, Marcucci R. Retinal vein thrombosis: risk factors, pathogenesis and therapeutic approach. Pathophysiology of Haemostasis & Thrombosis 2002;32(5‐6):308‐11. - PubMed
Raszewska‐Steglinska 2009
-
- Raszewska‐Steglinska M, Gozdek P, Cisiecki S, Michalewska Z, Michalewski J, Nawrocki J. Pars plana vitrectomy with ILM peeling for macular edema secondary to retinal vein occlusion. European Journal of Ophthalmology 2009;19(6):1055‐62. - PubMed
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rogers 2010
SCORE 2009
-
- Ip MS, Scott IU, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, et al. SCORE Study Research Group. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: The standard care vs corticosteroid for retinal vein occlusion (SCORE) study report 5. Archives of Ophthalmology 2009;127(9):1101‐14. - PMC - PubMed
Shahsuvaryan 2003
-
- Shahsuvaryan ML, Melkonyan AK. Central retinal vein occlusion risk profile: a case‐control study. European Journal of Ophthalmology 2003;13(5):445‐52. - PubMed
Shima 1996
-
- Shima DT, Gougos A, Miller JW, Tolentino M, Robinson G, Adamis AP, et al. Cloning and mRNA expression of vascular endothelial growth factor in ischemic retinas of Macaca fascicularis. Investigative Ophthalmology & Visual Science 1996;37(7):1334‐40. - PubMed
Stem 2013
Stewart 2008
-
- Stewart MW, Rosenfeld PJ. Predicted biological activity of intravitreal VEGF Trap. British Journal of Ophthalmology 2008;92(5):667‐8. - PubMed
Stewart 2012
-
- Stewart MW, Grippon S, Kirkpatrick P. Aflibercept. Nature Reviews. Drug Discovery 2012; Vol. 11, issue 4:269‐70. - PubMed
van der Reis 2011
-
- Reis MI, Heij EC, Jong‐Hesse Y, Ringens PJ, Hendrikse F, Schouten JS. A systematic review of the adverse events of intravitreal anti‐vascular endothelial growth factor injections. Retina (Philadelphia, Pa.) 2011;31(8):1449‐69. - PubMed
Vinores 1999
-
- Vinores SA, Derevjanik NL, Ozaki H, Okamoto N, Campochiaro PA. Cellular mechanisms of blood‐retinal barrier dysfunction in macular edema. Documenta Ophthalmologica 1999;97(3‐4):217‐28. - PubMed
Wittstrom 2012
-
- Wittstrom E, Holmberg H, Hvarfner C, Andreasson S. Clinical and electrophysiologic outcome in patients with neovascular glaucoma treated with and without bevacizumab. European Journal of Ophthalmology 2012;22(4):563‐74. - PubMed
Wolf‐Schnurrbusch 2011
-
- Wolf‐Schnurrbusch UE, Ghanem R, Rothenbuehler SP, Enzmann V, Framme C, Wolf S. Predictors of short‐term visual outcome after anti‐VEGF therapy of macular edema due to central retinal vein occlusion. Investigative Ophthalmology and Visual Science 2011;52(6):3334‐7. - PubMed
Wright 2007
-
- Wright JK, Franklin B, Zant E. Clinical case report: treatment of a central retinal vein occlusion with hyperbaric oxygen. Undersea & Hyperbaric Medicine 2007;34(5):315‐9. - PubMed
Wu 2008
-
- Wu L, Martinez‐Castellanos MA, Quiroz‐Mercado H, Arevalo JF, Berrocal MH, Farah ME, et al. Twelve‐month safety of intravitreal injections of bevacizumab (Avastin): results of the Pan‐American Collaborative Retina Study Group (PACORES). Graefes Archive for Clinical and Experimental Ophthalmology 2008;246(1):81‐7. - PubMed
Yasuda 2011
-
- Yasuda S, Kachi S, Kondo M, Ushida H, Uetani R, Terui T, et al. Significant correlation between electroretinogram parameters and ocular vascular endothelial growth factor concentration in central retinal vein occlusion eyes. Investigative Ophthalmology and Visual Science 2011;52(8):5737‐42. - PubMed
Zambarakji 2005
-
- Zambarakji HJ, Ghazi‐Nouri S, Schadt M, Bunce C, Hykin PG, Charteris DG. Vitrectomy and radial optic neurotomy for central retinal vein occlusion: effects on visual acuity and macular anatomy. Graefes Archive for Clinical and Experimental Ophthalmology 2005;243(5):397‐405. - PubMed
References to other published versions of this review
Braithwaite 2010
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

