Mucolytics for bronchiectasis
- PMID: 24789119
- PMCID: PMC6513404
- DOI: 10.1002/14651858.CD001289.pub2
Mucolytics for bronchiectasis
Abstract
Background: Bronchiectasis is predominantly an acquired disease process that represents the end stage of a variety of unrelated pulmonary insults. It is defined as persistent irreversible dilatation and distortion of medium-sized bronchi. It has been suggested that with widespread use of high-resolution computed tomography, more bronchiectasis diagnoses are being made. Patients diagnosed with bronchiectasis frequently have difficulty expectorating sputum. Sputum therefore is retained in the lungs and may become infected, leading to further lung damage. Mucolytic agents target hypersecretion or changed physiochemical properties of sputum to make it easier to clear. One drug, recombinant human DNase, breaks down the DNA that is released at the site of infection by neutrophils.Mucus clearance along with antimicrobial therapy remains an integral part of bronchiectasis management. Chest physiotherapy along with mucolytic agents is commonly used in practice without clear supportive evidence.
Objectives: To determine whether ingested or inhaled mucolytics are effective in the treatment of patients with bronchiectasis.
Search methods: We searched the Cochrane Airways Group Specialised Register and reference lists of relevant articles. We contacted experts in the field and drug companies. Searches were current as of June 2013.
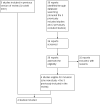
Selection criteria: Randomised trials of mucolytic treatment in people with bronchiectasis but not cystic fibrosis.
Data collection and analysis: Data extraction was performed independently by two review authors. Study authors were contacted for confirmation.
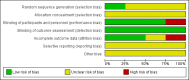
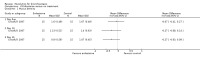
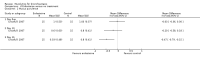
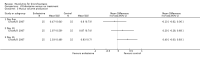
Main results: Four trials (with a combined total of 528 adult participants) were included, but almost none of the data from these studies could be aggregated in a meta-analysis.One trial (with 88 participants) compared bromhexine versus placebo. Compared with placebo, high doses of bromhexine with antibiotics eased difficulty in expectoration (mean difference (MD) -0.53, 95% confidence interval (CI) -0.81 to -0.25 at 16 days); the quality of the evidence was rated as low. A reduction in sputum production was noted with bromhexine (MD -21.5%, 95% CI -38.9 to -4.1 at day 16); again the quality of the evidence was rated as low. No significant differences between bromhexine and placebo were observed with respect to reported adverse events (odds ratio (OR) 2.93; 95% CI 0.12 to 73.97), and again the quality of the evidence was rated as low.In a single small, blinded but not placebo-controlled trial of older (> 55 years) participants with stable bronchiectasis and mucus hypersecretion, erdosteine combined with physiotherapy over a 15-day period improved spirometry and sputum purulence more effectively compared with physiotherapy alone. The spirometric improvement was small (MD 200 mL in forced expiratory volume in one second (FEV1) and 300 mL in forced vital capacity (FVC)) and was apparent only at day 15, not at earlier time points.The remaining two studies (with a combined total of 410 participants) compared recombinant human DNase (RhDNase) versus placebo. These two studies were very different (one was a two-week study of 61 participants, and the other ran for 24 weeks and included 349 participants), and the opportunity for combining data from the two studies was very limited. Compared with placebo, recombinant human DNase showed no difference in FEV1 or FVC in the smaller study but showed a significant negative effect on FEV1 in the larger and longer study. For reported adverse events, no significant differences between recombinant human DNase and placebo were noted. In all of the above comparisons of recombinant human DNase versus placebo, the quality of the evidence was judged to be low.
Authors' conclusions: Given the harmful effects of recombinant human DNase in one trial and no evidence of benefit, this drug should be avoided in non-cystic fibrosis bronchiectasis, except in the context of clinical trials. Evidence is insufficient to permit evaluation of the routine use of other mucolytics for bronchiectasis. High doses of bromhexine coupled with antibiotics may help with sputum production and clearance, but long-term data and robust clinical outcomes are lacking. Similarly, erdosteine may be a useful adjunct to physiotherapy in stable patients with mucus hypersecretion, but robust longer-term trials are required.Generally, clinical trials in children on the use of various mucolytic agents are lacking. As the number of agents available on the market, such as RhDNase, acetylcysteine and bromhexine, is increasing, improvement of the evidence base is needed.
Conflict of interest statement
None known.
Figures




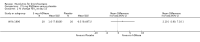
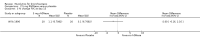

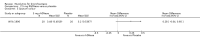



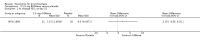
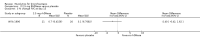

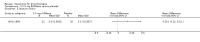

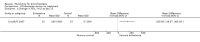
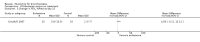
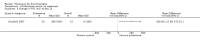
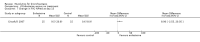
Update of
-
Mucolytics for bronchiectasis.Cochrane Database Syst Rev. 2001;(1):CD001289. doi: 10.1002/14651858.CD001289. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2014 May 02;(5):CD001289. doi: 10.1002/14651858.CD001289.pub2. PMID: 11279712 Updated.
References
References to studies included in this review
Crisafulli 2007 {published data only}
-
- Crisafulli E, Coletti O, Costi S, Zanasi E, Lorenzi C, Lucic S, et al. Effectiveness of erdosteine in elderly patients with bronchiectasis and hypersecretion: a 15‐day, prospective, parallel, open‐label, pilot study. Clinical Therapeutics 2007;29(9):2001‐9. - PubMed
O'Donnell 1998 {published data only}
-
- O'Donnell AE, Barker AF, Ilowite JS, Fick RB. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. rhDNase Study Group. Chest 1998;113(5):1329‐34. [] - PubMed
Olivieri 1991 {published data only}
-
- Olivieri D, Ciaccia A, Marangio E, Marsico S, Todisco T, Vita M. Role of bromhexine in exacerbations of bronchiectasis. Double‐blind randomized multicenter study versus placebo. Respiration 1991;58(3‐4):117‐21. [; 4900100000003559] - PubMed
Wills 1996 {published data only}
-
- Wills PJ, Wodehouse T, Corkery K, Mallon K, Wilson R, Cole PJ. Short‐term recombinant human DNase in bronchiectasis. Effect on clinical state and in vitro sputum transportability. American Journal of Respiratory and Critical Care Medicine 1996;154(2 Pt 1):413‐7. [; 4900100000005313] - PubMed
References to studies excluded from this review
Alberto 1968 {published data only}
-
- Alberto S, Colongo PG, Brusasco L, Frigerio G. [Studies of the clinical and respiratory functional effects of a mucolytic‐antibiotic preparation in chronic bronchopulmonary diseases. Controlled double‐blind single code studies] [Studi sugli effetti clinici e sulla funzionalità respiratoria di un preparato mucolitico‐antibiotico nelle affezioni croniche broncopolmonari. Ricerca controllata doppio‐cieca a codifica singola]. Minerva Medica 1968;59(53):2995‐3002. [] - PubMed
Balzano 1973 {published data only}
-
- Balzano E, Gaetani G. [D1‐sobrerol in the treatment of acute and chronic bronchopulmonary phlogoses]. [Italian] [Il dl sobrerolo nel trattamento delle flogosi broncopolmonari acute e croniche]. Minerva Medica 1973;64(37):1995‐2002. [] - PubMed
Bateman 1971 {published data only}
-
- Bateman PP. A new mucolytic, bromhexine ('bisolvon'). A double‐blind study. Medical Journal of Australia 1971;1(18):963‐5. [] - PubMed
Benjamin 1971 {published data only}
-
- Benjamin C. The use and efficacy of mucolytic agents. South African Medical Journal 1971; Vol. 45, issue 34:948‐52. [] - PubMed
Bergogne 1985 {published data only}
-
- Bergogne Berezin E, Berthelot G, Kafe HP, Dournovo P. Influence of a fluidifying agent (bromhexine) on the penetration of antibiotics into respiratory secretions. International Journal of Clinical Pharmacology Research 1985;5(5):341‐4. [] - PubMed
Bradley 2011 {published data only}
-
- Bradley JM, Treacy K, O'Neill B, McCourt F, Green L, Gardner E, et al. A randomised double blind 13 week crossover trial of hypertonic saline (HTS) (6%) vs isotonic saline (ITS) (0.9%) in patients with bronchiectasis [Abstract]. Thorax 2011;66(Suppl 4):A49 [S106]. []
Cobbin 1971 {published data only}
-
- Cobbin DM, Elliott FM, Rebuck AS. The mucolytic agent bromhexine (Bisolvon) in chronic lung disease: a double‐blind crossover trial. Australian and New Zealand Journal of Medicine 1971;2:137‐40. - PubMed
Currie 1988 {published data only}
Daviskas 1999 {published data only}
-
- Daviskas E, Anderson SD, Eberl S, Chan HK, Bautovich G, Anderson S, et al. Inhalation of dry powder mannitol improves clearance of mucus in patients with bronchiectasis. American Journal of Respiratory and Critical Care Medicine 1999;159(6):1843‐8. [; 4900100000008890] - PubMed
Fadda 2001 {published data only}
-
- Fadda G. Oral neltenexine in patients with obstructive airways diseases: an open, randomised, controlled comparison versus sobrerol. Minerva Medica 2001;92(4):269‐75. [; 4900100000011117] - PubMed
Germouty 1988 {published data only}
-
- Germouty J, Jirou‐Najou JL. Clinical trial of ambroxol in 2 different dosage programs in 120 patients with bronchiectasis. [Portuguese]. Revista Brasileira de Clinica E Terapeutica 1988;17(1‐2):33‐6. []
Ghiringhelli 1981 {published data only}
-
- Ghiringhelli G. Feprazone plus bromhexine in treatment of flare‐ups of chronic bronchopathies. [Italian]. Archivio Di Medicina Interna 1981;33(2):157‐68. []
Hasani 1994 {published data only}
-
- Hasani A, Pavia D, Spiteri MA, Yeo CT, Agnew JE, Clarke SW, et al. Regional mucus transport following unproductive cough and forced expiration technique in patients with airways obstruction. European Respiratory Journal 1994;105(5):1420‐5. [] - PubMed
Hasani 1994A {published data only}
Itoh 1984 {published data only}
-
- Itoh K, Kounou O, Morise M, Iwakura M, Misutani N, Katayama T, et al. Clinical effects of proteinase, sfericase (AI‐794), on chronic bronchitis and similar diseases. International Journal of Clinical Pharmacology, Therapy, and Toxicology 1984;22(1):32‐8. [] - PubMed
Kawashima 1989 {published data only}
-
- Kawashima K, Yamamoto H, Kadoya M, Kato M, Nata T, Iida E, et al. Clinical role of Branhamella catarrhalis infection in pulmonary disorders. [Japanese]. Saishin Igaku [Modern Medicine] 1989;44(6):1268‐72. []
Kellett 2005 {published data only}
-
- Kellett F, Redfern J, Niven RM, Kellet F. Evaluation of nebulised hypertonic saline (7%) as an adjunct to physiotherapy in patients with stable bronchiectasis. Respiratory Medicine 2005;99(1):27‐31. [] - PubMed
Kossmagk 1980 {published data only}
-
- Kossmagk R. Ultrasonic aerosol apparatus hire system ‐ Therapy of chronic obstructive bronchitis and bronchiectasia. [German]. Zeitschrift Fur Erkrankungen der Atmungsorgane 1980;155(1):125‐7. [] - PubMed
Loukides 1998 {published data only}
-
- Loukides S, Kharitonov S, Wodehouse T, Cole PJ, Barnes PJ. Effect of arginine on mucociliary function in primary ciliary dyskinesia. Lancet 1998;352(9125):371‐2. [; 4900100000023280] - PubMed
Mareels 1983 {published data only}
-
- Mareels J. A long term tolerance trial of bromhexine. Acta Therapeutica 1983;9(3):305‐15. []
Marthin 2007 {published data only}
-
- Marthin JK, Mortensen J, Pressler T, Nielsen KG. Pulmonary radioaerosol mucociliary clearance in diagnosis of primary ciliary dyskinesia. Chest 2007;132(3):966‐76. [; 4900100000021265] - PubMed
Noone 1999 {published data only}
-
- Noone PG, Bennett WD, Regnis JA, Zeman KL, Carson JL, King M, et al. Effect of aerosolized uridine‐5'‐triphosphate on airway clearance with cough in patients with primary ciliary dyskinesia. American Journal of Respiratory and Critical Care Medicine 1999;160(1):144‐9. [; 4900100000006450] - PubMed
Patterson 2007 {published data only}
-
- Patterson JE, Hewitt O, Kent L, Bradbury I, Elborn JS, Bradley JM. Acapella versus 'usual airway clearance' during acute exacerbation in bronchiectasis: a randomized crossover trial. Chronic Respiratory Disease 2007;4(2):67‐74. [] - PubMed
Sahay 1982 {published data only}
-
- Sahay JN, Chatterjee SS, Ingram DF. The effect of methyl cysteine (Visclair) in respiratory diseases. A pilot study. Clinical Trials Journal 1982;19(3):137‐43. []
Serisier 2013 {published data only}
-
- Serisier DJ, Martin ML, McGuckin MA, Lourie R, Chen AC, Brain B, et al. Effect of long‐term, low‐dose erythromycin on pulmonary exacerbations among patients with non‐cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA 2013; Vol. 309, issue 12:1260‐7. - PubMed
Stafanger 1988 {published data only}
-
- Stafanger G, Garne S, Howitz P, Morkassel E, Koch C. The clinical effect and the effect on the ciliary motility of oral N‐acetylcysteine in patients with cystic fibrosis and primary ciliary dyskinesia. The European Respiratory Journal 1988;1(2):161‐7. [; 4900100000002512] - PubMed
Tambascio 2011 {published data only}
-
- Tambascio J, Souza LT, Lisboa RM, Passarelli RDCV, Souza HCD, Gastaldi AC. The influence of Flutter VRP1 components of mucus transport of patients with bronchiectasis. Respiratory Medicine 2011;105(9):1316‐21. [] - PubMed
Taskar 1992 {published data only}
-
- Taskar VS, Sharma RR, Goswami R, John PJ, Mahashur AA. Effect of bromhexine on sputum amoxycillin levels in lower respiratory infections. Respiratory Medicine 1992;86(2):157‐60. [; 4900100000003765] - PubMed
Tsang 2003 {published data only}
-
- Tsang SMH, Jones AYM, Tsang SM H, Jones AYM. Postural drainage or flutter device in conjunction with breathing and coughing compared to breathing and coughing alone in improving secretion removal and lung function in patients with acute exacerbation of bronchiectasis. A pilot study. Hong Kong Physiotherapy Journal 2003;21:29‐36. []
Verstraeten 1979 {published data only}
-
- Verstraeten JM. Mucolytic treatment in chronic obstructive lung disease: double‐blind comparative clinical trial with N‐acetylcysteine, bromhexine and placebo. Acta Tuberculosea et Pneumologica Belgica 1979;70(1):71‐80. - PubMed
Wong 2012 {published data only}
-
- Wong C, Jayaram L, Karalus N, Eaton T, Tong C, Hockey H, et al. Azithromycin for prevention of exacerbations in non‐cystic. Lancet 2012;380(9842):660‐7. [] - PubMed
Yalçin 2006 {published data only}
-
- Yalçin E, Kiper N, Ozçelik U, Doğru D, Firat P, Sahin A, et al. Effects of clarithromycin on inflammatory parameters and clinical conditions in children with bronchiectasis. Journal of Clinical Pharmacy and Therapeutics 2006;31(1):49‐55. [] - PubMed
Additional references
Balsamo 2010
BTS 2010
-
- Pasteur MC, Bilton D, Hill AT. BTS guideline for non‐CF bronchiectasis. www.brit‐thoracic.org.uk/LinkClick.aspx?link=496&tabid=69 (accessed 15 November 2013).
Cotgreave 1987
-
- Cotgreave I, Eklund A, Larsson K, Moldéus P. No penetration of orally administered N‐acetylcysteine into bronchoalveolar lavage fluid. European Journal of Respiratory Diseases 1987;70(2):73‐7. - PubMed
Goeminne 2010
-
- Goeminne P, Dupont L. Non‐cystic fibrosis bronchiectasis: diagnosis and management in 21st century. Postgraduate Medical Journal 2010;86:493‐501. - PubMed
Henke 2007
-
- Henke MO, Ratjen F. Mucolytics in cystic fibrosis. Paediatric Respiratory Reviews 2007;8(1):24–9. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Review Manager (RevMan) [Computer program]
-
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rogers 2007
-
- Rogers DF. Mucoactive agents for airway mucus hypersecretory diseases. Respiratory Care 2007;52(9):1176–97. - PubMed
Stafler 2010
-
- Stafler P, Carr S. Non cystic fibrosis bronchiectasis: its diagnosis and management. Archives of Diseases in Childhood. Education and Practice Edition 2010;95:73‐82. - PubMed
Stockley 1995
-
- Stockley RA. Role of inflammation in respiratory tract infections. American Journal of Medicine 1995;99(6):8S–13S. - PubMed
Tomkiewicz 1995
-
- Tomkiewicz RP, App EM, Sanctis GT, Coffiner M, Maes P, Rubin BK, et al. A comparison of a new mucolytic N‐acetylcysteine l‐lysinate with N‐acetylcysteine: airway epithelial function and mucus changes in dog. Pulmonary Pharmacology 1995;8(6):259–65. - PubMed
TSANZ 2010
-
- TSANZ and the Lung Foundation. Chronic suppurative lung disease and bronchiectasis in children and adults 2010. http://www.lungfoundation.com.au/professional‐resources/guidelines/ (accessed 15 November 2013).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

