Simultaneous pharmacokinetic modeling of gentamicin, tobramycin and vancomycin clearance from neonates to adults: towards a semi-physiological function for maturation in glomerular filtration
- PMID: 24789450
- PMCID: PMC4749758
- DOI: 10.1007/s11095-014-1361-z
Simultaneous pharmacokinetic modeling of gentamicin, tobramycin and vancomycin clearance from neonates to adults: towards a semi-physiological function for maturation in glomerular filtration
Erratum in
-
Correction: "Simultaneous Pharmacokinetic Modeling of Gentamicin, Tobramycin and Vancomycin Clearance From Neonates to Adults: Towards a Semi-physiological Function for Maturation in Glomerular Filtration".Pharm Res. 2025 Aug;42(8):1459. doi: 10.1007/s11095-025-03895-3. Pharm Res. 2025. PMID: 40696262 No abstract available.
Abstract
Purpose: Since glomerular filtration rate (GFR) is responsible for the elimination of a large number of water-soluble drugs, the aim of this study was to develop a semi-physiological function for GFR maturation from neonates to adults.
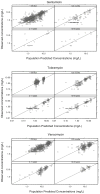
Methods: In the pharmacokinetic analysis (NONMEM VI) based on data of gentamicin, tobramycin and vancomycin collected in 1,760 patients (age 1 day-18 years, bodyweight 415 g-85 kg), a distinction was made between drug-specific and system-specific information. Since the maturational model for clearance is considered to contain system-specific information on the developmental changes in GFR, one GFR maturational function was derived for all three drugs.
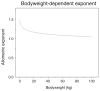
Results: Simultaneous analysis of these three drugs showed that maturation of GFR mediated clearance from preterm neonates to adults was best described by a bodyweight-dependent exponent (BDE) function with an exponent varying from 1.4 in neonates to 1.0 in adults (ClGFR = Cldrug*(BW/4 kg)(BDE) with BDE = 2.23*BW(-0.065)). Population clearance values (Cldrug) for gentamicin, tobramycin and vancomycin were 0.21, 0.28 and 0.39 L/h for a full term neonate of 4 kg, respectively.
Discussion: Based on an integrated analysis of gentamicin, tobramycin and vancomycin, a semi-physiological function for GFR mediated clearance was derived that can potentially be used to establish evidence based dosing regimens of renally excreted drugs in children.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

