Addition of CF3 across unsaturated moieties: a powerful functionalization tool
- PMID: 24789472
- PMCID: PMC4141301
- DOI: 10.1039/c4cs00025k
Addition of CF3 across unsaturated moieties: a powerful functionalization tool
Abstract
In the last few years, the efficient introduction of trifluoromethyl groups in organic molecules has become a major research focus. This review highlights the recent developments enabling the incorporation of CF3 groups across unsaturated moieties, preferentially alkenes, and the mechanistic scenarios governing these transformations. We have specially focused on methods involving the simultaneous formation of C-CF3 and C-C or C-heteroatom bonds by formal addition reactions across π-systems, as such difunctionalization processes hold valuable synthetic potential.
Figures


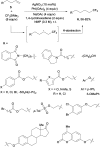
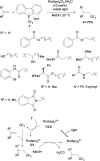

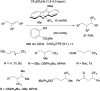

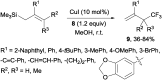
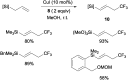
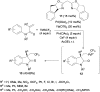





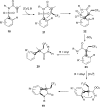
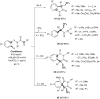

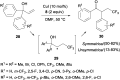




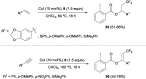
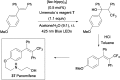


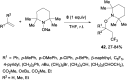

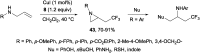

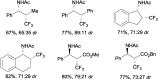
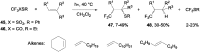


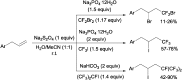
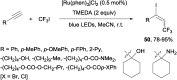
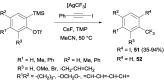


References
-
- Kirsch P., Modern Fluoroorganic Chemistry: Synthesis Reactivity Applications, Wiley-VCH, Weinheim, 2004.
-
- Yamazaki T., Taguchi T. and Ojima I., Fluorine in Medicinal Chemistry and Chemical Biology, Wiley-Blackwell, Chichester, Great Britain, 2009.
-
- Shibata N., Mizuta S., Kawai H. Tetrahedron: Asymmetry. 2008;19:2633–2644.
-
- Tomashenko O. A., Grushin V. V. Chem. Rev. 2011;111:4475–4521. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

