MicroRNA-486-dependent modulation of DOCK3/PTEN/AKT signaling pathways improves muscular dystrophy-associated symptoms
- PMID: 24789910
- PMCID: PMC4038577
- DOI: 10.1172/JCI73579
MicroRNA-486-dependent modulation of DOCK3/PTEN/AKT signaling pathways improves muscular dystrophy-associated symptoms
Abstract
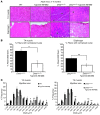
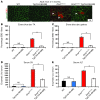
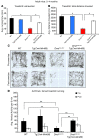
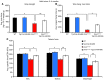
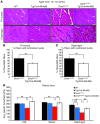
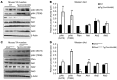
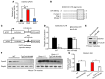
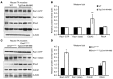
Duchenne muscular dystrophy (DMD) is caused by mutations in the gene encoding dystrophin, which results in dysfunctional signaling pathways within muscle. Previously, we identified microRNA-486 (miR-486) as a muscle-enriched microRNA that is markedly reduced in the muscles of dystrophin-deficient mice (Dmdmdx-5Cv mice) and in DMD patient muscles. Here, we determined that muscle-specific transgenic overexpression of miR-486 in muscle of Dmdmdx-5Cv mice results in reduced serum creatine kinase levels, improved sarcolemmal integrity, fewer centralized myonuclei, increased myofiber size, and improved muscle physiology and performance. Additionally, we identified dedicator of cytokinesis 3 (DOCK3) as a miR-486 target in skeletal muscle and determined that DOCK3 expression is induced in dystrophic muscles. DOCK3 overexpression in human myotubes modulated PTEN/AKT signaling, which regulates muscle hypertrophy and growth, and induced apoptosis. Furthermore, several components of the PTEN/AKT pathway were markedly modulated by miR-486 in dystrophin-deficient muscle. Skeletal muscle-specific miR-486 overexpression in Dmdmdx-5Cv animals decreased levels of DOCK3, reduced PTEN expression, and subsequently increased levels of phosphorylated AKT, which resulted in an overall beneficial effect. Together, these studies demonstrate that stable overexpression of miR-486 ameliorates the disease progression of dystrophin-deficient skeletal muscle.
Figures

References
-
- Cirak S, et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet. 2011;378(9791):595–605. doi: 10.1016/S0140-6736(11)60756-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

