Alcohol abuse: critical pathophysiological processes and contribution to disease burden
- PMID: 24789985
- PMCID: PMC4046814
- DOI: 10.1152/physiol.00055.2013
Alcohol abuse: critical pathophysiological processes and contribution to disease burden
Abstract
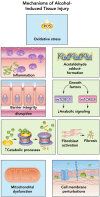
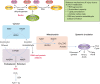
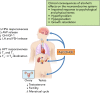
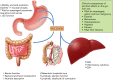
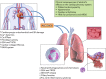
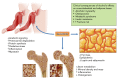
Alcohol abuse; the most common and costly form of drug abuse, is a major contributing factor to many disease categories. The alcohol-attributable disease burden is closely related to the average volume of alcohol consumption, with dose-dependent relationships between amount and duration of alcohol consumption and the incidence of diabetes mellitus, hypertension, cardiovascular disease, stroke, and pneumonia. The frequent occurrence of alcohol use disorders in the adult population and the significant and widespread detrimental organ system effects highlight the importance of recognizing and further investigating the pathophysiological mechanisms underlying alcohol-induced tissue and organ injury.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
Figures
References
-
- Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology 108: 218–224, 1995 - PubMed
-
- Addolorato G, Capristo E, Marini M, Santini P, Scognamiglio U, Attilia ML, Messineo D, Sasso GF, Gasbarrini G, Ceccanti M. Body composition changes induced by chronic ethanol abuse: evaluation by dual energy X-ray absorptiometry. Am J Gastroenterol 95: 2323–2327, 2000 - PubMed
-
- Aguilar D, Skali H, Moyé LA, Lewis EF, Gaziano JM, Rutherford JD, Hartley LH, Randall OS, Geltman EM, Lamas GA, Rouleau JL, Pfeffer MA, Solomon SD. Alcohol consumption and prognosis in patients with left ventricular systolic dysfunction after a myocardial infarction. J Am Coll Cardiol 43: 2015–2021, 2004 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

