The aging mouse partially models the aging human spine: lumbar and coccygeal disc height, composition, mechanical properties, and Wnt signaling in young and old mice
- PMID: 24790018
- PMCID: PMC4064379
- DOI: 10.1152/japplphysiol.01322.2013
The aging mouse partially models the aging human spine: lumbar and coccygeal disc height, composition, mechanical properties, and Wnt signaling in young and old mice
Abstract
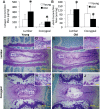
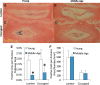
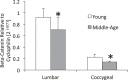
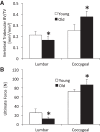
Murine lumbar and coccygeal (tail) regions of spines are commonly used to study cellular signaling of age-related disc diseases, but the tissue-level changes of aging intervertebral discs and vertebrae of each spinal region remain unclear. Furthermore, the impact of aging lumbar and coccygeal discs on Wnt/β-catenin signaling, which is putatively involved in the catabolism of intervertebral discs, is also unclear. We compared disc/vertebrae morphology and mechanics and biochemical composition of intervertebral discs from lumbar and coccygeal regions between young (4-5 mo) and old (20-22 mo) female C57BL/6 mice. Center intervertebral disc height from both regions was greater in old discs than young discs. Compared with young, old lumbar discs had a lower early viscous coefficient (a measure of stiffness) by 40%, while conversely old coccygeal discs were stiffer by 53%. Biochemically, old mice had double the collagen content in lumbar and coccygeal discs of young discs, greater glycosaminoglycan in lumbar discs by 37%, but less glycosaminoglycan in coccygeal discs by 32%. Next, we compared Wnt activity of lumbar and coccygeal discs of 4- to 5-mo and 12- to 14-mo TOPGAL mice. Despite the disc-specific changes, aging decreased Wnt signaling in the nucleus pulposus from both spinal regions by ≥64%. Compared with young, trabecular bone volume/tissue volume and ultimate force were less in old lumbar vertebrae, but greater in old coccygeal vertebrae. Thus intervertebral discs and vertebrae age in a spinal region-dependent manner, but these differential age-related changes may be uncoupled from Wnt signaling. Overall, lumbar and coccygeal regions are not interchangeable in modeling human aging.
Keywords: WNT/β-catenin; aging; caudal; mouse; tail.
Copyright © 2014 the American Physiological Society.
Figures
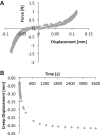
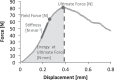
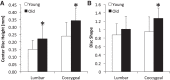
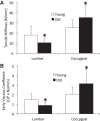
References
-
- Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest 98: 996–1003, 1996 - PMC - PubMed
-
- Beckstein JC, Sen S, Schaer TP, Vresilovic EJ, Elliott DM. Comparison of animal discs used in disc research to human lumbar disc: axial compression mechanics and glycosaminoglycan content. Spine 33: E166–E173, 2008 - PubMed
-
- Blaney-Davidson EN, Vitters EL, vandenBerg WB, van der Kraan PM. Rapid decrease in Smad2/3 signaling in the annulus fibrosus co-incides with loss of lamellar structure in the aging murine intervetebral discs, while Smad1/58P stays stable until 16 months. In: ORS 2012 Meeting (Online). http://www.ors.org/Transactions/58/2175.pdf: 2012 [5 December 2013].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

