Administration of recombinant soluble urokinase receptor per se is not sufficient to induce podocyte alterations and proteinuria in mice
- PMID: 24790179
- PMCID: PMC4116049
- DOI: 10.1681/ASN.2013040425
Administration of recombinant soluble urokinase receptor per se is not sufficient to induce podocyte alterations and proteinuria in mice
Abstract
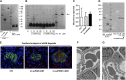
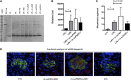
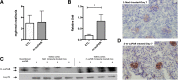
Circulating levels of soluble forms of urokinase-type plasminogen activator receptor (suPAR) are generally elevated in sera from children and adults with FSGS compared with levels in healthy persons or those with other types of kidney disease. In mice lacking the gene encoding uPAR, forced increases in suPAR concentration result in FSGS-like glomerular lesions and proteinuria. However, whether overexpression of suPAR, per se, contributes to the pathogenesis of FSGS in humans remains controversial. We conducted an independent set of animal experiments in which two different and well characterized forms of recombinant suPAR produced by eukaryotic cells were administered over the short or long term to wild-type (WT) mice. In accordance with the previous study, the delivered suPARs are deposited in the glomeruli. However, such deposition of either form of suPAR in the kidney did not result in increased glomerular proteinuria or altered podocyte architecture. Our findings suggest that glomerular deposits of suPAR caused by elevated plasma levels are not sufficient to engender albuminuria.
Keywords: glomerulosclerosis; nephrotic syndrome; pathophysiology of renal disease and progression; podocyte; proteinuria.
Copyright © 2014 by the American Society of Nephrology.
Figures
Comment in
-
Soluble urokinase-type plasminogen activator receptor in FSGS: stirred but not shaken.J Am Soc Nephrol. 2014 Aug;25(8):1611-3. doi: 10.1681/ASN.2014030257. Epub 2014 May 1. J Am Soc Nephrol. 2014. PMID: 24790180 Free PMC article. No abstract available.
-
Glomerular disease: The search goes on: suPAR is not the elusive FSGS factor.Nat Rev Nephrol. 2014 Aug;10(8):431-2. doi: 10.1038/nrneph.2014.113. Epub 2014 Jun 24. Nat Rev Nephrol. 2014. PMID: 24957131 No abstract available.
References
-
- Hoyer JR, Vernier RL, Najarian JS, Raij L, Simmons RL, Michael AF: Recurrence of idiopathic nephrotic syndrome after renal transplantation. Lancet 2: 343–348, 1972 - PubMed
-
- Le Berre L, Godfrin Y, Perretto S, Smit H, Buzelin F, Kerjaschki D, Usal C, Cuturi C, Soulillou JP, Dantal J: The Buffalo/Mna rat, an animal model of FSGS recurrence after renal transplantation. Transplant Proc 33: 3338–3340, 2001 - PubMed
-
- Wei C, Möller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP, Cowan P, Kretzler M, Parrilla R, Bendayan M, Gupta V, Nikolic B, Kalluri R, Carmeliet P, Mundel P, Reiser J: Modification of kidney barrier function by the urokinase receptor. Nat Med 14: 55–63, 2008 - PubMed
-
- Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M, Zhang Q, Nikolic B, Chaudhuri A, Daftarian P, Salido E, Torres A, Salifu M, Sarwal MM, Schaefer F, Morath C, Schwenger V, Zeier M, Gupta V, Roth D, Rastaldi MP, Burke G, Ruiz P, Reiser J: Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med 17: 952–960, 2011 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

