GPR179 is required for high sensitivity of the mGluR6 signaling cascade in depolarizing bipolar cells
- PMID: 24790204
- PMCID: PMC4004817
- DOI: 10.1523/JNEUROSCI.4044-13.2014
GPR179 is required for high sensitivity of the mGluR6 signaling cascade in depolarizing bipolar cells
Abstract
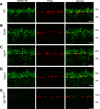
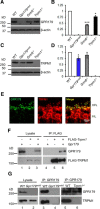
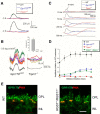
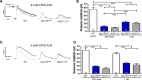
Parallel visual pathways are initiated at the first retinal synapse by signaling between the rod and cone photoreceptors and two general classes of bipolar cells. For normal function, ON or depolarizing bipolar cells (DBCs) require the G-protein-coupled receptor, mGluR6, an intact G-protein-coupled cascade and the transient receptor potential melastatin 1 (TRPM1) cation channel. In addition, another seven transmembrane protein, GPR179, is required for DBC function and recruits the regulators of G-protein signaling (RGS) proteins, RGS7 and RGS11, to the dendritic tips of the DBCs. Here we use the Gpr179(nob5) mouse, which lacks GPR179 and has a no b-wave electroretinogram (ERG) phenotype, to demonstrate that despite the absence of both GPR179 and RGS7/RGS11, a small dark-adapted ERG b-wave remains and can be enhanced with long duration flashes. Consistent with the ERG, the mGluR6-mediated gating of TRPM1 can be evoked pharmacologically in Gpr179(nob5) and RGS7(-/-)/RGS11(-/-) rod BCs if strong stimulation conditions are used. In contrast, direct gating of TRPM1 by capsaicin in RGS7(-/-)/RGS11(-/-) and WT rod BCs is similar, but severely compromised in Gpr179(nob5) rod BCs. Noise and standing current analyses indicate that the remaining channels in Gpr179(nob5) and RGS7(-/-)/RGS11(-/-) rod BCs have a very low open probability. We propose that GPR179 along with RGS7 and RGS11 controls the ability of the mGluR6 cascade to gate TRPM1. In addition to its role in localizing RGS7 and RGS11 to the dendritic tips, GPR179 via a direct interaction with the TRPM1 channel alters its ability to be gated directly by capsaicin.
Keywords: GPR179; TRPM1; mGluR6; night blindness; retina; rod bipolar.
Figures


References
-
- Audo I, Kohl S, Leroy BP, Munier FL, Guillonneau X, Mohand-Saïd S, Bujakowska K, Nandrot EF, Lorenz B, Preising M, Kellner U, Renner AB, Bernd A, Antonio A, Moskova-Doumanova V, Lancelot ME, Poloschek CM, Drumare I, Defoort-Dhellemmes S, Wissinger B, et al. TRPM1 is mutated in patients with autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet. 2009;85:720–729. doi: 10.1016/j.ajhg.2009.10.013. - DOI - PMC - PubMed
-
- Audo I, Bujakowska K, Orhan E, Poloschek CM, Defoort-Dhellemmes S, Drumare I, Kohl S, Luu TD, Lecompte O, Zrenner E, Lancelot ME, Antonio A, Germain A, Michiels C, Audier C, Letexier M, Saraiva JP, Leroy BP, Munier FL, Mohand-Saïd S, et al. Whole-exome sequencing identifies mutations in GPR179 leading to autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet. 2012;90:321–330. doi: 10.1016/j.ajhg.2011.12.007. - DOI - PMC - PubMed
-
- Boycott BB, Dowling JE, Kolb H. Organization of the primate retina: light microscopy. Philos Trans R Soc Lond B Biol Sci. 1969;255:109–184. doi: 10.1098/rstb.1969.0004. - DOI
-
- Cao Y, Masuho I, Okawa H, Xie K, Asami J, Kammermeier PJ, Maddox DM, Furukawa T, Inoue T, Sampath AP, Martemyanov KA. Retina-specific GTPase accelerator RGS11/G beta 5S/R9AP is a constitutive heterotrimer selectively targeted to mGluR6 in ON-bipolar neurons. J Neurosci. 2009;29:9301–9313. doi: 10.1523/JNEUROSCI.1367-09.2009. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
