Sleep fragmentation in mice induces nicotinamide adenine dinucleotide phosphate oxidase 2-dependent mobilization, proliferation, and differentiation of adipocyte progenitors in visceral white adipose tissue
- PMID: 24790279
- PMCID: PMC3985100
- DOI: 10.5665/sleep.3678
Sleep fragmentation in mice induces nicotinamide adenine dinucleotide phosphate oxidase 2-dependent mobilization, proliferation, and differentiation of adipocyte progenitors in visceral white adipose tissue
Abstract
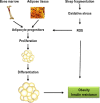
Background: Chronic sleep fragmentation (SF) without sleep curtailment induces increased adiposity. However, it remains unclear whether mobilization, proliferation, and differentiation of adipocyte progenitors (APs) occurs in visceral white adipose tissue (VWAT), and whether nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 2 (Nox2) activity plays a role.
Methods: Changes in VWAT depot cell size and AP proliferation were assessed in wild-type and Nox2 null male mice exposed to SF and control sleep (SC). To assess mobilization, proliferation, and differentiation of bone marrow mesenchymal stem cells (BM-MSC), Sca-1+ bone marrow progenitors were isolated from GFP+ or RFP+ mice, and injected intravenously to adult male mice (C57BL/6) previously exposed to SF or SC.
Results: In comparison with SC, SF was associated with increased weight accrual at 3 w and thereafter, larger subcutaneous and visceral fat depots, and overall adipocyte size at 8 w. Increased global AP numbers in VWAT along with enhanced AP BrDU labeling in vitro and in vivo emerged in SF. Systemic injections of GFP+ BM-MSC resulted in increased AP in VWAT, as well as in enhanced differentiation into adipocytes in SF-exposed mice. No differences occurred between SF and SC in Nox2 null mice for any of these measurements.
Conclusions: Chronic sleep fragmentation (SF) induces obesity in mice and increased proliferation and differentiation of adipocyte progenitors (AP) in visceral white adipose tissue (VWAT) that are mediated by increased Nox2 activity. In addition, enhanced migration of bone marrow mesenchymal stem cells from the systemic circulation into VWAT, along with AP differentiation, proliferation, and adipocyte formation occur in SF-exposed wild-type but not in oxidase 2 (Nox2) null mice. Thus, Nox2 may provide a therapeutic target to prevent obesity in the context of sleep disorders.
Keywords: adipocyte progenitor cells; adipose tissue; obesity; oxidative stress; sleep fragmentation.
Figures






References
-
- Rosekind MR. Underestimating the societal costs of impaired alertness: safety, health and productivity risks. Sleep Med. 2005:S21–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

