Biological responses according to the shape and size of carbon nanotubes in BEAS-2B and MESO-1 cells
- PMID: 24790438
- PMCID: PMC4000181
- DOI: 10.2147/IJN.S58661
Biological responses according to the shape and size of carbon nanotubes in BEAS-2B and MESO-1 cells
Abstract
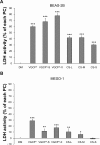
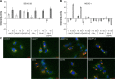
This study aimed to investigate the influence of the shape and size of multi-walled carbon nanotubes (MWCNTs) and cup-stacked carbon nanotubes (CSCNTs) on biological responses in vitro. Three types of MWCNTs - VGCF(®)-X, VGCF(®)-S, and VGCF(®) (vapor grown carbon fibers; with diameters of 15, 80, and 150 nm, respectively) - and three CSCNTs of different lengths (CS-L, 20-80 μm; CS-S, 0.5-20 μm; and CS-M, of intermediate length) were tested. Human bronchial epithelial (BEAS-2B) and malignant pleural mesothelioma cells were exposed to the CNTs (1-50 μg/mL), and cell viability, permeability, uptake, total reactive oxygen species/superoxide production, and intracellular acidity were measured. CSCNTs were less toxic than MWCNTs in both cell types over a 24-hour exposure period. The cytotoxicity of endocytosed MWCNTs varied according to cell type/size, while that of CSCNTs depended on tube length irrespective of cell type. CNT diameter and length influenced cell aggregation and injury extent. Intracellular acidity increased independently of lysosomal activity along with the number of vacuoles in BEAS-2B cells exposed for 24 hours to either CNT (concentration, 10 μg/mL). However, total reactive oxygen species/superoxide generation did not contribute to cytotoxicity. The results demonstrate that CSCNTs could be suitable for biological applications and that CNT shape and size can have differential effects depending on cell type, which can be exploited in the development of highly specialized, biocompatible CNTs.
Keywords: cup-stacked carbon nanotube; cytotoxicity; in vitro; intracellular acidity; multi-walled carbon nanotube.
Figures




References
-
- Saito N, Usui Y, Aoki K, et al. Carbon nanotubes: biomaterial applications. Chem Soc Rev. 2009;38(7):1897–1903. - PubMed
-
- Li X, Fan Y, Watari F. Current investigations into carbon nanotubes for biomedical application. Biomed Mater. 2010;5(2):22001. - PubMed
-
- Adams D, Williams DF. The response of bone to carbon – carbon composites. Biomaterials. 1984;5(2):59–64. - PubMed
-
- Bokros JC. Carbon biomedical devices. Carbon. 1977;15(6):353–371.
-
- Poland CA, Duffin R, Kinloch I, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3(7):423–428. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

