Experience in the use of clobazam in the treatment of Lennox-Gastaut syndrome
- PMID: 24790647
- PMCID: PMC3994921
- DOI: 10.1177/1756285614521314
Experience in the use of clobazam in the treatment of Lennox-Gastaut syndrome
Abstract
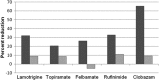
Clobazam is a 1,5-benzodiazepine used successfully worldwide since the 1970s as an anxiolytic and antiepileptic drug. Since its recent Food and Drug Administration (FDA) approval in the United States in 2011 as adjunctive treatment for Lennox-Gastaut syndrome, it has continued to show sustained efficacy and a better safety and tolerability profile compared with other benzodiazepines. The two randomized, controlled studies that led to the US FDA approval, as well as the follow-up multicenter, open-label study of clobazam, showed ≥50% seizure reduction for more than 50% of Lennox-Gastaut syndrome patients, while none of the other FDA-approved treatments for LGS have demonstrated efficacy rates better than 50%. Clobazam appears to have a safe profile and sustained effectiveness over the first 3 years of use in LGS and other epilepsy syndromes with intractable seizures, which makes it a viable long-term treatment option.
Keywords: Lennox–Gastaut syndrome; benzodiazepine; clobazam; drop seizures; open-label trial; pediatric epilepsy.
Conflict of interest statement
Figures


References
-
- Arzimanoglou A., French J., Blume W., Cross J., Ernst J., Feucht M., et al. (2009) Lennox–Gastaut syndrome: a consensus approach on diagnosis, assessment, management, and trial methodology. Lancet Neurol 8: 82–93 - PubMed
-
- Canadian Clobazam Cooperative Group (1991) Clobazam in treatment of refractory epilepsy: the Canadian experience. A retrospective study. Epilepsia 32: 407–416 - PubMed
-
- Commission on Classification and Terminology of the International League Against Epilepsy (1989) Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 30: 389–399 - PubMed
-
- Conry J., Ng Y., Paolicchi J., Kernitsky L., Mitchell W., Ritter F., et al. (2009) Clobazam in the treatment of Lennox–Gastaut syndrome. Epilepsia 50: 1158–1166 - PubMed
-
- Cramer J., Sapin C., François C. (2013) Indirect comparison of clobazam and other therapies for Lennox–Gastaut syndrome. Acta Neurol Scand 128: 91–99 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

