Comparative study of in vitro release and mucoadhesivity of gastric compacts composed of multiple unit system/bilayered discs using direct compression of metformin hydrochloride
- PMID: 24790896
- PMCID: PMC4005280
- DOI: 10.5681/bi.2014.002
Comparative study of in vitro release and mucoadhesivity of gastric compacts composed of multiple unit system/bilayered discs using direct compression of metformin hydrochloride
Abstract
Introduction: Metformin is an oral anti-diabetic drug in the biguanide class. The goal of this study was to develop gastric-retentive MH discs in order to prolong the retention of drug in gastric mucosa.
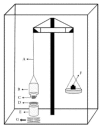
Methods: Two groups of metformin hydrochloride (MH) mucoadhesive gastroretentive discs were prepared: (a) bilayered discs prepared by direct compression of powders containing polymers as Carbopol 934P (CP, mucoadhesive polymer) and ethylcellulose (EC, rotardant polymer), (b) multiple unit system (microparticle) discs prepared by the emulsification, solvent evaporation, and compression technique from microparticles using polymers CP and EC. Gastric-mucoadhesive compacts were evaluated by investigating their release pattern, swelling capacity, mucoadhesion property, surface pH, and in vitro gastro-retentive time. Discs formulation was subjected to disintegration and dissolution tests by placing in 0.1 M hydrochloric acid for 8 h.
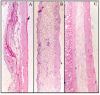
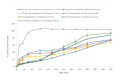
Results: The production yield showed F2 microparticles of 98.80%, mean particle size of 933.25 µm and loading efficiency of 98.44%. The results showed that prepared microparticle discs had slower release than bilayered discs (p>0.05). The bilayered discs exhibited very good percentage of mucoadhesion. The results also showed a significant higher retention of mucoadhesive bilayered discs in upper gastrointestinal tract (F´1, 1:2 ratio of CP:EC). Histopathological studies revealed no gastric mucosal damage.
Conclusion: Mucoadhesive multiple unit system/bilayered discs interact with mucus of gastrointestinal tract and are considered to be localized or trapped at the adhesive site by retaining a dosage form at the site of action as well as improving in the intimacy of contact with underlying absorptive membrane to achieve a better therapeutic performance of anti-diabetic drug.
Keywords: Bilayered discs; Carbomer 934P; Ethylcellulose; Metformin hydrochloride; Multiple unit system discs.
Similar articles
-
Formulation and Evaluation of In-vitro Characterization of Gastic-Mucoadhesive Microparticles/Discs Containing Metformin Hydrochloride.Iran J Pharm Res. 2014 Winter;13(1):67-80. Iran J Pharm Res. 2014. PMID: 24734057 Free PMC article.
-
Design of a novel bilayered gastric mucoadhesive system for localized and unidirectional release of lamotrigine.Saudi Pharm J. 2013 Jan;21(1):45-52. doi: 10.1016/j.jsps.2012.01.004. Epub 2012 Jan 28. Saudi Pharm J. 2013. PMID: 24109205 Free PMC article.
-
Formulation and Evaluation of Propranolol Hydrochloride-Loaded Carbopol-934P/Ethyl Cellulose Mucoadhesive Microspheres.Iran J Pharm Res. 2010 Summer;9(3):221-32. Iran J Pharm Res. 2010. PMID: 24363731 Free PMC article.
-
Formulation and evaluation of stomach-specific amoxicillin-loaded carbopol-934P mucoadhesive microspheres for anti-Helicobacter pylori therapy.J Microencapsul. 2009 Jun;26(4):365-76. doi: 10.1080/02652040802373012. J Microencapsul. 2009. PMID: 18720199
-
Novel mucoadhesion tests for polymers and polymer-coated particles to design optimal mucoadhesive drug delivery systems.Adv Drug Deliv Rev. 2005 Nov 3;57(11):1583-94. doi: 10.1016/j.addr.2005.07.008. Epub 2005 Sep 16. Adv Drug Deliv Rev. 2005. PMID: 16169120 Review.
Cited by
-
Formulation and in vitro evaluation of a fast-disintegrating/sustained dual release bucoadhesive bilayer tablet of captopril for treatment of hypertension crises.Res Pharm Sci. 2016 Jul;11(4):274-83. doi: 10.4103/1735-5362.189284. Res Pharm Sci. 2016. PMID: 27651807 Free PMC article.
-
An Overview of Microparticulate Drug Delivery System and its Extensive Therapeutic Applications in Diabetes.Adv Pharm Bull. 2022 Aug;12(4):730-746. doi: 10.34172/apb.2022.075. Epub 2021 Oct 4. Adv Pharm Bull. 2022. PMID: 36415632 Free PMC article. Review.
-
Long non-coding RNAs as potential biomarkers or therapeutic targets in gastric cancer.Gastroenterol Hepatol Bed Bench. 2023;16(3):297-306. doi: 10.22037/ghfbb.v16i2.2701. Gastroenterol Hepatol Bed Bench. 2023. PMID: 37767321 Free PMC article.
-
Local Drug Delivery Systems as Novel Approach for Controlling NETosis in Periodontitis.Pharmaceutics. 2024 Sep 6;16(9):1175. doi: 10.3390/pharmaceutics16091175. Pharmaceutics. 2024. PMID: 39339210 Free PMC article. Review.
References
-
- Smart JD. The basics and underlying mechanisms of mucoadhesion. Advanced drug delivery reviews. 2005;57:1556–68. - PubMed
-
- Junginger HE, Thanou M Verhoef JC,. Drug Delivery: Mucoadhesive Hydrogels. In: Swarbrick S, editor. Encyclopedia of Pharmaceutical Technology. New York: CRC Press;2002. p. 1848-63.
-
- Lee JW, Park JH, Robinson JR. Bioadhesive-based dosage forms: The next generation. J Pharm Sci. 2000;89:850–66. - PubMed
-
- Hägerstrom H, Edsman K. Limitations of the rheological mucoadhesion method: the effect of the choice of conditions and the rheological synergism parameter. Eur J Pharm Sci. 2003;18:349–57. - PubMed
-
- Sharma HK, Pradhan SP, Sarangi B. Preparation and in-vitro evaluation of enteric controlled release pantoprazole loaded microbeads using natural mucoadhesive substance from Dillenia Indica L. Int J Pharm Tech Res. 2010;2:542–51.
LinkOut - more resources
Full Text Sources
Miscellaneous