Harmonization: the sample, the measurement, and the report
- PMID: 24790905
- PMCID: PMC3999316
- DOI: 10.3343/alm.2014.34.3.187
Harmonization: the sample, the measurement, and the report
Abstract
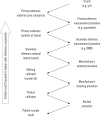
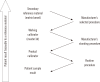
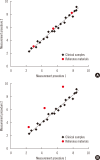
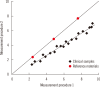
Harmonization of clinical laboratory results means that results are comparable irrespective of the measurement procedure used and where or when a measurement was made. Harmonization of test results includes consideration of pre-analytical, analytical, and post-analytical aspects. Progress has been made in each of these aspects, but there is currently poor coordination of the effort among different professional organizations in different countries. Pre-analytical considerations include terminology for the order, instructions for preparation of the patient, collection of the samples, and handling and transportation of the samples to the laboratory. Key analytical considerations include calibration traceability to a reference system, commutability of reference materials used in a traceability scheme, and specificity of the measurement of the biomolecule of interest. International organizations addressing harmonization include the International Federation for Clinical Chemistry and Laboratory Medicine, the World Health Organization, and the recently formed International Consortium for Harmonization of Clinical Laboratory Results (ICHCLR). The ICHCLR will provide a prioritization process for measurands and a service to coordinate global harmonization activities to avoid duplication of effort. Post-analytical considerations include nomenclature, units, significant figures, and reference intervals or decision values for results. Harmonization in all of these areas is necessary for optimal laboratory service. This review summarizes the status of harmonization in each of these areas and describes activities underway to achieve the goal of fully harmonized clinical laboratory testing.
Keywords: Commutability; Harmonization; Standardization; Traceability.
Figures

References
-
- Sacks DB. In: Tietz textbook of clinical chemistry and molecular diagnostics. 5th eds. Burtis CA, Ashwood ER, Bruns DE, editors. Philadelphia: Elsevier; 2012. p. 719.
-
- Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Kirkman MS, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2011;57:e1–e47. - PubMed
-
- Wu AHB. Tietz clinical guide to laboratory tests. 4th ed. St. Louis: WB Saunders; 2006.
-
- Clinical and Laboratory Standards Institute. Expression of measurement uncertainty in laboratory medicine; Approved Guideline. Wayne, PA: Clinical and Laboratory Standards Institute; 2012. CLSI document EP29-A.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

