Multiple Functional Motifs Are Required for the Tumor Suppressor Activity of a Constitutively-Active ErbB4 Mutant
- PMID: 24791013
- PMCID: PMC4002051
Multiple Functional Motifs Are Required for the Tumor Suppressor Activity of a Constitutively-Active ErbB4 Mutant
Abstract
ErbB4 (HER4) is a member of the ErbB family of receptor tyrosine kinases, which includes the Epidermal Growth Factor Receptor (EGFR/ErbB1), ErbB2 (HER2/Neu), and ErbB3 (HER3). Mounting evidence indicates that ErbB4, unlike EGFR or ErbB2, functions as a tumor suppressor in many human malignancies. Previous analyses of the constitutively-dimerized and -active ErbB4 Q646C mutant indicate that ErbB4 kinase activity and phosphorylation of ErbB4 Tyr1056 are both required for the tumor suppressor activity of this mutant in human breast, prostate, and pancreatic cancer cell lines. However, the cytoplasmic region of ErbB4 possesses additional putative functional motifs, and the contributions of these functional motifs to ErbB4 tumor suppressor activity have been largely underexplored. Here we demonstrate that ErbB4 BH3 and LXXLL motifs, which are thought to mediate interactions with Bcl family proteins and steroid hormone receptors, respectively, are required for the tumor suppressor activity of the ErbB4 Q646C mutant. Furthermore, abrogation of the site of ErbB4 cleavage by gamma-secretase also disrupts the tumor suppressor activity of the ErbB4 Q646C mutant. This last result suggests that ErbB4 cleavage and subcellular trafficking of the ErbB4 cytoplasmic domain may be required for the tumor suppressor activity of the ErbB4 Q646C mutant. Indeed, here we demonstrate that mutants that disrupt ErbB4 kinase activity, ErbB4 phosphorylation at Tyr1056, or ErbB4 cleavage by gamma-secretase also disrupt ErbB4 trafficking away from the plasma membrane and to the cytoplasm. This supports a model for ErbB4 function in which ErbB4 tumor suppressor activity is dependent on ErbB4 trafficking away from the plasma membrane and to the cytoplasm, mitochondria, and/or the nucleus.
Keywords: ErbB4/HER4; Protein Trafficking; Signal Transduction; Tumor Suppressor.
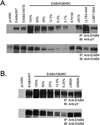
Figures







Similar articles
-
The Yin and Yang of ERBB4: Tumor Suppressor and Oncoprotein.Pharmacol Rev. 2022 Jan;74(1):18-47. doi: 10.1124/pharmrev.121.000381. Pharmacol Rev. 2022. PMID: 34987087 Free PMC article. Review.
-
Ligand stimulation of ErbB4 and a constitutively-active ErbB4 mutant result in different biological responses in human pancreatic tumor cell lines.Exp Cell Res. 2011 Feb 15;317(4):392-404. doi: 10.1016/j.yexcr.2010.11.007. Epub 2010 Nov 24. Exp Cell Res. 2011. PMID: 21110957 Free PMC article.
-
Phosphorylation of ErbB4 on Tyr1056 is critical for inhibition of colony formation by prostate tumor cell lines.Biochem Biophys Res Commun. 2006 Oct 13;349(1):372-82. doi: 10.1016/j.bbrc.2006.08.055. Epub 2006 Aug 18. Biochem Biophys Res Commun. 2006. PMID: 16934755 Free PMC article.
-
Constitutively active ErbB4 and ErbB2 mutants exhibit distinct biological activities.Cell Growth Differ. 2002 Jun;13(6):247-56. Cell Growth Differ. 2002. PMID: 12114214
-
ErbB Receptors and Cancer.Methods Mol Biol. 2017;1652:3-35. doi: 10.1007/978-1-4939-7219-7_1. Methods Mol Biol. 2017. PMID: 28791631 Review.
Cited by
-
The Yin and Yang of ERBB4: Tumor Suppressor and Oncoprotein.Pharmacol Rev. 2022 Jan;74(1):18-47. doi: 10.1124/pharmrev.121.000381. Pharmacol Rev. 2022. PMID: 34987087 Free PMC article. Review.
-
Fatty acid synthase affects expression of ErbB receptors in epithelial to mesenchymal transition of breast cancer cells and invasive ductal carcinoma.Oncol Lett. 2017 Nov;14(5):5934-5946. doi: 10.3892/ol.2017.6954. Epub 2017 Sep 15. Oncol Lett. 2017. PMID: 29113229 Free PMC article.
-
HER4 Affects Sensitivity to Tamoxifen and Abemaciclib in Luminal Breast Cancer Cells and Restricts Tumor Growth in MCF-7-Based Humanized Tumor Mice.Int J Mol Sci. 2024 Jul 8;25(13):7475. doi: 10.3390/ijms25137475. Int J Mol Sci. 2024. PMID: 39000582 Free PMC article.
References
-
- Riese DJ, 2nd, Stern DF. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays. 1998;20:41–48. - PubMed
-
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. - PubMed
-
- Elenius K, Corfas G, Paul S, Choi CJ, Rio C, et al. A novel juxtamembrane domain isoform of HER4/ErbB4. Isoform-specific tissue distribution and differential processing in response to phorbol ester. J Biol Chem. 1997;272:26761–26768. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
