Multimodal analysis of the progression of Best vitelliform macular dystrophy
- PMID: 24791142
- PMCID: PMC4000718
Multimodal analysis of the progression of Best vitelliform macular dystrophy
Abstract
Purpose: To investigate the multimodal morphological features in the different stages of Best vitelliform macular dystrophy (VMD) in subjects harboring mutations in the BEST1 gene, and their changes during the progression of the disease.
Methods: In this retrospective observational study performed between January 2007 and December 2012, 21 patients (42 eyes) with Best VMD from eight families with the BEST1 mutation were included. Best-corrected visual acuity (BCVA), fundus autofluorescence (FAF), and spectral domain optical coherence tomography (SDOCT) were evaluated at study entry and at last visit.
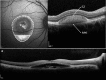
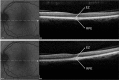
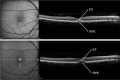
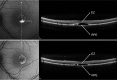
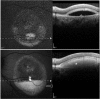
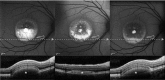
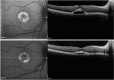
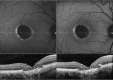
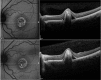
Results: The mean age of patients was 26.3±17.4 years. Seven new missense mutations in BEST1 were identified. Mean follow-up was 41.1±18.5 months. Mean BCVA was 0.34±0.34 LogMAR at study entry and 0.32±0.33 LogMAR at last follow-up visit (p = 0.2). The overall lesion area on FAF increased from 6.62±4.9 mm² to 7.34±6.1 mm² (p = 0.05). At study entry, on SD-OCT, photoreceptor inner segment ellipsoid portion (ellipsoid zone, EZ) was normal in 15 eyes, disrupted in 14 eyes, and absent in 13 eyes. In two eyes, EZ changed from normal to disrupted during follow-up. Three eyes of three patients showing pseudohypopyon lesions at study entry progressed to vitelliruptive lesions at the last follow-up visit. Three eyes of three patients showing vitelliruptive lesion at study entry reverted to pseudohypopyon lesion with overall enlargement of the lesion size.
Conclusions: Multimodal analysis allowed documenting a continuous material accumulation and reabsorption in Best VMD progression. Blue FAF and SD-OCT could represent noninvasive imaging techniques to monitor Best VMD.
Figures
Similar articles
-
Functional assessment of the fundus autofluorescence pattern in Best vitelliform macular dystrophy.Graefes Arch Clin Exp Ophthalmol. 2016 Jul;254(7):1297-302. doi: 10.1007/s00417-015-3194-9. Epub 2015 Oct 21. Graefes Arch Clin Exp Ophthalmol. 2016. PMID: 26490373
-
Functional and clinical data of Best vitelliform macular dystrophy patients with mutations in the BEST1 gene.Mol Vis. 2009 Dec 31;15:2960-72. Mol Vis. 2009. PMID: 20057903 Free PMC article.
-
The spectrum of subclinical Best vitelliform macular dystrophy in subjects with mutations in BEST1 gene.Invest Ophthalmol Vis Sci. 2011 Jun 28;52(7):4678-84. doi: 10.1167/iovs.10-6500. Invest Ophthalmol Vis Sci. 2011. PMID: 21436265
-
Near-infrared fundus autofluorescence in subclinical best vitelliform macular dystrophy.Am J Ophthalmol. 2014 Dec;158(6):1247-1252.e2. doi: 10.1016/j.ajo.2014.08.028. Epub 2014 Aug 28. Am J Ophthalmol. 2014. PMID: 25174897
-
Best Vitelliform Macular Dystrophy.Adv Exp Med Biol. 2025;1467:87-91. doi: 10.1007/978-3-031-72230-1_16. Adv Exp Med Biol. 2025. PMID: 40736818 Review.
Cited by
-
The pseudohypopyon stage in adult-onset foveomacular vitelliform dystrophy.Int Ophthalmol. 2023 Sep;43(9):3107-3113. doi: 10.1007/s10792-023-02710-5. Epub 2023 Apr 17. Int Ophthalmol. 2023. PMID: 37067692
-
Retinal Imaging Findings in Inherited Retinal Diseases.J Clin Med. 2024 Apr 3;13(7):2079. doi: 10.3390/jcm13072079. J Clin Med. 2024. PMID: 38610844 Free PMC article. Review.
-
[Multimodal imaging and evaluation in the age of artificial intelligence].Ophthalmologe. 2020 Oct;117(10):965-972. doi: 10.1007/s00347-020-01210-6. Ophthalmologe. 2020. PMID: 32845382 Review. German.
-
Gene therapy in bestrophinopathies: Insights from preclinical studies in preparation for clinical trials.Saudi J Ophthalmol. 2023 Dec 1;37(4):287-295. doi: 10.4103/sjopt.sjopt_175_23. eCollection 2023 Oct-Dec. Saudi J Ophthalmol. 2023. PMID: 38155675 Free PMC article.
-
SPACR Encoded by IMPG1 Is Essential for Photoreceptor Survival by Interplaying between the Interphotoreceptor Matrix and the Retinal Pigment Epithelium.Genes (Basel). 2022 Aug 23;13(9):1508. doi: 10.3390/genes13091508. Genes (Basel). 2022. PMID: 36140676 Free PMC article.
References
-
- Best F. Uber eine hereditare maculaafektion; Beitrage zur verergslehre. Zschr Augenheilkunde. 1905;13:199–212.
-
- Stone EM, Nichols BE, Streb LM, Kimura AE, Sheffield VC. Genetic linkage of vitelliform macular degeneration Best's disease to chromosome 11q13. Nat Genet. 1992;1:246–50. - PubMed
-
- Petrukhin K, Koisti MJ, Bakall B, Li W, Xie G, Marknell T, Sandgren O, Forsman K, Holmgren G, Andreasson S, Vujic M, Bergen AA, McGarty-Dugan V, Figueroa D, Austin CP, Metzker ML, Caskey CT, Wadelius C. Identification of the gene responsible for Best macular dystrophy. Nat Genet. 1998;19:241–7. - PubMed
-
- Kaufman SJ, Goldberg MF, Orth DH, Fishman GA, Tessler H, Mizuno K. Autosomal dominant vitreoretinochoroidopathy. Arch Ophthalmol. 1982;100:272–8. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

