Effects of synaptic plasticity on phase and period locking in a network of two oscillatory neurons
- PMID: 24791223
- PMCID: PMC4003516
- DOI: 10.1186/2190-8567-4-8
Effects of synaptic plasticity on phase and period locking in a network of two oscillatory neurons
Abstract
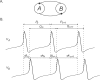
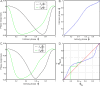
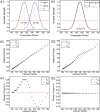
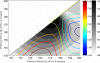
We study the effects of synaptic plasticity on the determination of firing period and relative phases in a network of two oscillatory neurons coupled with reciprocal inhibition. We combine the phase response curves of the neurons with the short-term synaptic plasticity properties of the synapses to define Poincaré maps for the activity of an oscillatory network. Fixed points of these maps correspond to the phase-locked modes of the network. These maps allow us to analyze the dependence of the resulting network activity on the properties of network components. Using a combination of analysis and simulations, we show how various parameters of the model affect the existence and stability of phase-locked solutions. We find conditions on the synaptic plasticity profiles and the phase response curves of the neurons for the network to be able to maintain a constant firing period, while varying the phase of locking between the neurons or vice versa. A generalization to cobwebbing for two-dimensional maps is also discussed.
Keywords: Oscillatory neural network; Phase locking; Phase response curve; Short-term synaptic plasticity.
Figures







References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

